 полная версия
полная версияPorcelain
The greater infusibility that accompanies this hardness was not a point of much importance to them, but they marvelled at the translucency of the edges, as of some natural stone, and we find absurdly exaggerated accounts of the transparency both of the original ware and of the imitation that they claimed to have made. Finally, they noticed that the whiteness of the surface was not given by an artificial layer more or less closely adhering to an earthy base, but was the natural colour of the paste to which the thin layer of transparent glaze merely gave the effect of the polish on ivory or on marble. What then was this hard, white, translucent substance? What wonder if from one end of Europe to the other, scheming minds—chemists, alchemists, physicians, potters, and charlatans—were at work trying to make something that should resemble it? The history of this long search is a very interesting one, but it would be impossible to explain its failures, its partial failures (these last resulting in a compromise—soft-paste porcelain), and the final success of Böttger, without, as it were, going behind the scenes, and giving some account of porcelain from a modern, scientific point of view.
And first let us say that, although when treating of porcelain from the historical and especially from the æsthetic standpoint (and this after all is our principal business in this book), it is well to take a wide grasp and include a whole class of china—I mean the soft-paste ware—which does not come up to our standard of hardness and infusibility, this is not the case when we are considering the physical, and especially the chemical, nature of porcelain. By confining ourselves, for the present, to true hard porcelain, we have the advantage of dealing with a substance which chemically and physically may be compared to a definite mineral species. Nay more, we propose here to confine ourselves to the consideration of the hard pastes used at the present day in the wares of France and Germany, neglecting for the present the softer and more irregular porcelain of the Chinese.
First as regards hardness, the surface of the paste of a true porcelain, when free from glaze, can be scratched by a crystal of quartz, but it is untouched by the hardest steel. That is to say, it would be classed by the mineralogist with felspar, and given a hardness of 6 to 6·5 on his scale.1
The freshly broken edge shows a white, perfectly uniform substance, a glassy or vitreous lustre, a finely granular texture, and a fracture conchoidal to splintery. When struck, a vessel of porcelain gives a clear, bell-like note, and in this differs from other kinds of pottery. When held against the light it allows, where the piece is sufficiently thin, a certain amount to pass through, but even in the thinnest splinters porcelain is never transparent.
If a thin section be made of a piece of porcelain, and this be examined under the microscope by transmitted light, we see, scattered in a clear, or nearly clear, paste, a vast number of minute, slender rods, and between them many minute granules (Church’s English Porcelain, p. 6). These belonites and spherulites, as they have been called, doubtless reflect the light which would otherwise pass through the glassy base in which they float, and the partial reflection and partial transmission of the light may not be unconnected with the lustrous fracture so characteristic of porcelain. Their presence points to the fact that we are dealing with a more or less definite substance, one which may be compared to a natural mineral species, and not merely with a semi-fused clay, something between stoneware and glass. Now when we come to treat of the chemical constitution of porcelain, we shall find that this view is confirmed. This structure is developed in the paste by the exposure, for a considerable period of time, to a temperature of from 1300° to 1500° centigrade, a temperature which is sufficient to reduce all other kinds of pottery, with the exception of some kinds of stoneware, to a glassy mass. In the case of porcelain, this great and prolonged heat allows of a complete rearrangement of the molecules in the softened mass. The process may be compared to that by which certain minerals and rocks are formed in the depths of the earth.
We see, then, that not only from the standpoint of history, but on the basis of the physical properties and intimate constitution of the material, we are able to draw a sharp line between porcelain and other fictile wares. This distinction is even more definitely shown by a chemical analysis.2
We are dealing, as in the case of so large a part of the rocks and minerals of the earth’s surface, with certain silicates of the alkalis and alkaline earths, with silicates of alumina above all. All natural clays used for fictile purposes consist essentially of silicates of various bases, such as alumina, lime, iron, potash, and soda, more or less intimately combined with water, and with the addition, generally, of some free silica. If the clay be good in working quality and colour, the next point the potter has to look to is the question of its fusibility. It may be said generally that the simpler the constitution of a silicate, that is the smaller the number of bases that it contains, the greater will be its resistance to fire. Silicate of alumina is unaltered at 1500° C., a temperature which may be taken as the maximum at the command of the potter. The fusing-point is reduced by the addition of silica, especially if some other bases such as oxide of iron or lime, or again an alkali, are present even in small quantity. But beyond a certain point the addition of silica raises the fusing-point, and it is important to note that it is this excess of silica that renders certain stonewares and fire-clays so infusible. In the case of porcelain, on the other hand, the resistance to high temperatures depends more upon the percentage of alumina present, and the absence or small amount of other bases. Thus in comparing the composition of different porcelains, we find that it is those that contain the most silica that are the most fusible, or rather, to speak more accurately, that become ‘porcelainised’ at a lower temperature.3
The relation of porcelain to stoneware on the one hand, and to ordinary pottery on the other, will be made clear by the following figures, which give the composition of stoneware, Meissen porcelain, and of a red Samian ware:—
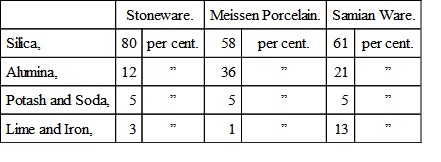
The refractory stoneware contains a large excess of silica over the amount required to combine with the alumina and the ‘other bases.’ In the easily fusible Roman pottery, the ‘other bases’ nearly equal in amount the alumina, while the Meissen porcelain not only contains less silica than the pottery, but the ‘other bases’ only amount to a sixth part of the alumina present.
But it is not enough for the manufacturer to discover a clay of which the chemical composition corresponds to that of the type of porcelain which he proposes to make. The question, as an experiment of Brongniart long ago proved, is more complicated. Brongniart weighed out the separate constituents for his porcelain—the silica, the alumina, and the alkalis—and from them he formed his paste. He found, however, that the paste readily melted at the heat of the porcelain furnace. The analysis then of any ceramic product can give us but an imperfect clue to the nature and properties of the ware. We want to know how the elements are arranged, and this can only be inferred from a knowledge of the materials employed in the manufacture. I will illustrate this point by comparing the composition of Meissen porcelain with that of our Dorsetshire pipe-clay, the most famous of our English clays, but a material not sufficiently refractory for use in the manufacture of porcelain. Both substances contain the same amount of alumina—36 per cent.; in the Poole clay (after removing the water) there is 55 per cent. of silica and 9 per cent. of ‘other bases,’ against 58 per cent. and 6 per cent. respectively in the porcelain. The composition, therefore, of the two bodies is nearly the same: the clay, while it contains more iron-oxide and lime than the porcelain, is poorer in silica.
True porcelain has indeed never been made from any other materials than those so long employed by the Chinese and first described by the missionary, Père D’Entrecolles, nearly two hundred years ago.
The two essential elements in the composition of porcelain are—(a) The hydrated silicate of alumina, which is provided by the white earthy clay known as kaolin or china-clay, a substance infusible at the highest temperature attainable by our furnaces (about 1500° C.); (b) The silicate of alumina and potash (or more rarely soda), that is to say felspar. But the felspar is generally associated with some amount of both quartz and mica, and is itself in a more or less disintegrated condition. This is the substance known as petuntse or china-stone. It is fusible at the higher temperatures of the porcelain kiln.
Of those substances the first is an immediate product of the weathering of the felspar contained in granitic rocks; while the second, the petuntse, is nothing else than the granite (or allied rock) itself in a more or less weathered condition.
We see, then, that speaking generally, granite is the source of both the materials whose intimate mixture in the state of the finest comminution constitutes the paste of porcelain. It thus happens that it is only in regions of primitive rocks, far away as a rule from centres of industry and indeed from the usual sources of the clay used for fictile ware, that the materials essential for making porcelain are found. By the term granite we mean here a crystalline rock consisting of felspar, quartz, and mica, and we include in the term gneiss, which differs only in the arrangement of its constituents. The many varieties of rock that are named as sources of kaolin and petuntse, such as pegmatite, graphic granite, or growan-stone, are as a rule varieties of granite4 distinguished by containing little or no mica, and above all by the absence of iron in appreciable quantity. As felspar is also the sole or at least the principal element in the glaze with which porcelain is covered, it will be seen that it is the mineral with which we are above all concerned.
Now, of the three minerals that enter into the constitution of these granitic rocks (the others are quartz and mica), felspar is the one most easily acted on by air and water. The carbonic acid which is always present in the surface-water gradually removes the alkaline constituents in the form of soluble carbonates, the silicate of alumina which remains takes up and combines with a certain quantity of water, and in this form it is washed down into hollows to form the beds of white crumbly clay known as kaolin. This is, of course, a somewhat general and theoretical statement of what happens. If we were to examine the actual position and geological relation to the surrounding rocks of the beds of kaolin in Cornwall and in the south-west of France, there might be some exceptions to be made and difficulties to explain. Where, indeed, as in many places in Cornwall, the kaolinisation has extended to great depth, the decomposition may have been caused by deep-seated agencies; in such cases the kaolin is often associated with minerals containing fluorine and boron.5
As for the other constituent of porcelain, the petuntse or china-stone, we have called it a disintegrated granite, and this is the condition in which it is usually excavated. It corresponds to the French cailloux, the stony or gravelly material as opposed to the clay. In French works it is not generally distinguished from felspar, and indeed some varieties of petuntse may contain little else. However, if pure felspar is used, the second constituent in granite or in petuntse, I mean quartz, will have to be added to our porcelain paste in the form of sand or powdered flint. The third constituent of the china-stone, the mica, is usually neglected: in many cases the mother rock contains but little, and what there is is eliminated in the washing. Mica is more fusible than felspar; the white variety, muscovite, is practically free from iron, and only from granite rocks containing this variety can petuntse suitable for the manufacture of porcelain be obtained. The importance of mica as an element of the Chinese petuntse has only recently been recognised (Vogt, Comptes Rendus, 1890, p. 43). As much as 40 per cent. of muscovite has been found in samples brought from China. The pegmatite of the Limoges district, on the other hand, contains only 30 per cent. of this white mica, and of this only a small portion passes into the paste. We have here, perhaps, the principal cause of the greater hardness and the higher softening-point of European compared with Oriental porcelain.
We shall see later on that this softer Chinese paste has many advantages, especially in its relation to the glaze and the enamels, but for the present we will continue to take the more ‘severe’ European porcelain as our type.
Let us consider what takes place during the firing of a paste of this latter description. After all the water, including that in combination in the kaolin, has been driven off, we have, as the temperature rises, an intimate mixture of two silicates, one of which, if heated alone, would be unaltered by any temperature at our command—this is the silicate of alumina derived from the kaolin; while the other is a fusible silicate of alumina and potash. There is also present a certain amount of free silica. There is reason to believe that at a certain point a chemical reaction takes place between these constituents, accompanied by a local rapid rise of temperature in the materials, the rise being due to this reaction. As a result there is a rearrangement of the molecules of the mass, although no complete fusion takes place. It is now, says M. Vernadsky (Comptes Rendus, 1890, p. 1377)—we are now following the account of his experiments—that the sub-crystalline rods—the baculites of which we have already spoken—are formed. M. Vernadsky claims to have separated these rods from the glassy base by means of hydrofluoric acid, in which the former were insoluble. He found them to consist of a very basic silicate of alumina, containing as much as 70 per cent. of that earth, while the glassy base was chiefly composed of silica in combination with the potash and with a small quantity of alumina. In their optical properties the crystals or baculites resemble the mineral known as sillimanite, a natural silicate of alumina.
This is all that scientific research has so far been able to tell us of the intimate constitution of porcelain; but as far as it goes, it is evidence in favour of our claim that we are dealing with a definite substance, sui generis, and not merely with a casual mixture of certain superior kinds of clay, something, as we have said, between glass and stoneware.
There are certain other elements that enter at times into the composition of porcelain—magnesia, which may have been added to the paste in the form either of steatite or magnesite; and lime, derived either from gypsum or chalk. These additions generally tend to increase the fusibility of the paste, especially when accompanied by an additional dose of silica; but as their presence is not essential we are not concerned with these substances here.
The glazes used for porcelain are as a rule distinguished by their comparative infusibility and by their containing no lead. The composition of these glazes follows more or less that of the paste that they cover, with such modifications, however, as to allow of a somewhat lower fusing-point: as in the case of the paste, there is a harder and more refractory, and a softer and more fusible, type. The harder glazes are composed essentially of felspar, with the addition in most cases of silica, kaolin, and powdered fragments of porcelain. At Sèvres, a natural rock, pegmatite, consisting chiefly of felspar, has been melted to form a glaze without further addition. Of late years, however, the introduction of a milder type of porcelain has necessitated the use of a more fusible glaze, containing a considerable quantity of lime, and it is a glaze of this latter type that has with few exceptions found favour in other districts where porcelain is made.
We have attempted in this chapter to give some idea of the nature of porcelain from a physical and chemical point of view, and in doing so have taken as our type the hard, refractory paste of Europe. When we come to describe the porcelain of the Chinese, we shall notice some important divergences from this type. We say nothing here of the soft-paste porcelains, seeing that so long as we confine ourselves to the question of chemical composition and physical properties, they lie entirely outside our definitions. It is only from the point of view of its history and of its artistic qualities that this group has any claim to the name of porcelain.
CHAPTER II
THE MATERIALS: MIXING, FASHIONING, AND FIRING
IT would be quite foreign to the scope and object of this book to attempt to describe in any detail the different processes that come into play in the manufacture of a piece of porcelain. There is the less cause for any such detailed treatment, inasmuch as the operations involved in the preparation of the paste and in the subsequent potting and firing do not essentially differ in the case of porcelain from those employed in the manufacture of other classes of pottery. The differences are rather those of degree—greater care is necessary in the selection of the materials, and these materials must be more finely ground and more intimately mixed. Again, the great heat required in the kilns necessitates, in the firing of porcelain, many precautions that are not called for in the case of earthenware or fayence. Without, however, some slight acquaintance with the processes of the manufacture, it would be impossible to avoid an amateurish and somewhat ‘anecdotal’ treatment of our subject. There are, indeed, many intimate features, many delicate shades of difference that distinguish the wares of various times and places, both in Europe and in the East, which can only be rationally explained by reference to the details of the manufacture.
At the present day there is only one district in Europe where true porcelain is manufactured on a large scale. This district lies on the western and south-western border of the central granitic plateau of France, especially in the Limousin and in Berry. Again at Sèvres, for the last hundred years and more, a succession of able chemists has carried on a series of experiments on the composition and preparation of porcelain. It is no wonder, then, if we find that the literature concerned with these practical departments is almost entirely French. One result of this is a greater richness in technical terms than with us. We find in France names for the various implements and processes of the potter’s art, that are something better than the workshop terms of the local potter. Again, the little that has been written in England upon the technology of pottery has been concerned chiefly with earthenware of Staffordshire.6
As for the English soft-paste porcelain of the eighteenth century, there is a remarkable dearth of information both as to its composition and as to its manufacture. We know in fact in much greater detail how the great potteries at King-te-chen were carried on at the same period, thanks to the letters of the Père D’Entrecolles, and to the information collected in Dr. Bushell’s great work, Oriental Ceramic Art (New York, 1899. I shall always quote from the text edition).
The following technical notes are based chiefly on the processes in use either at Sèvres or in the great factories of the Limoges district.7 To begin with the Kaolin, the ‘premier’ element in the composition of porcelain. The greatest care is taken to procure a pure white clay which should approach as near as possible to the more or less theoretical mineral kaolinite, i.e. to a hydrous silicate of alumina. With this object the rough china-clay brought from the pit is thrown into a large tank of water and broken up with wooden spades; the milky liquid is now decanted into a second tank, leaving behind most of the quartz and the other stony particles. On its way the soup-like liquid passes through the meshes of a sieve—these may be formed either of brass wire or sometimes of finely woven silk. On this sieve all but the finest particles are retained. The greater part of the kaolin is deposited in this second tank, but a certain portion still remains suspended in the liquid, which is again decanted; the remaining kaolin then settles down in the third tank, yielding the finest clay. To dry this slimy mass, it is first forced by hydraulic pumps into canvas bags, and these bags are then pressed between fluted wooden trays, strongly clamped together. We have now got a white chalky mass which may contain as much as 98 per cent. of the hydrated silicate of alumina.
The other materials, the china-stone8 and the quartz, have first to be reduced to the finest powder. To effect this they may, to begin with, be roasted to effect disintegration, then crushed in a stone-breaking machine, and finally passed through the grinding-pan in which they are ground fine between large blocks of chert which rotate upon a pavement of the same stone. The finely ground materials have now to be mixed in suitable proportions either by the old process of ‘slop-blending,’ where the different ‘slops,’ each of known specific gravity, are run in due proportion into the big ‘blending ark,’ or, as is now usual in the case of fine wares, by weighing out the materials in a dry state. On the relative amounts of the three elements, the china-clay, the china-stone, and the quartz, the nature of the porcelain after firing will depend. M. Vogt (La Porcelaine, Paris, 1893) gives a useful table showing the limits within which the materials may be varied. We may note that in the case of a normal china-stone or petuntse being used instead of felspar, very little additional quartz is required. These limits are: kaolin, 35 to 65 per cent.; felspar, 20 to 40 per cent.; and quartz, 15 to 25 per cent. The larger the percentage of the first material, the harder and more refractory will be the resultant porcelain.
This question of the composition of the paste has been the subject of many experiments lately at Sèvres. A somewhat animated discussion has raged around it. M. Vogt, who is the director of the technical department in the National Porcelain Works, is well qualified to speak on the subject. We shall not hesitate then to avail ourselves of the conclusions which he arrives at, the more so as they put tersely some important points of which we shall see the importance later on. I refer especially to the relations of the glazes and the coloured decorations to the subjacent paste.
These are, then, the results that M. Vogt arrives at:—
The two extreme types of porcelain, one with 65 per cent. of kaolin and the other with only 35 per cent., when taken from the kiln do not differ in appearance, though one has been subject to a temperature of 1500° C. to ensure vitrification and the other to only 1350° C. Their physical properties, however, are very different. The first, rich in alumina derived from the excess of kaolin, stands without injury variations of temperature, it suits well with a glaze made from felspar, a glaze hard enough to resist the point of a knife. These are excellent qualities for domestic use, but such porcelain does not lend itself well to artistic decoration. At the high temperature required in this case in the firing, the colours of the paste and of the glazes assume dull and tame hues, so as to offer little resource to the artist. In a word, in that part of the decoration that has to be subjected to the full heat of the kiln, the artist has command only of a restricted and relatively dull palette. Again, in the decoration of the muffle-stove the vitrifiable enamels do not become incorporated with the glaze on which they rest. If a decoration in opaque or translucent enamels is attempted, these enamels are apt to split off, carrying with them a part of the glaze. To sum up: the porcelain of which the hard paste of Sèvres, introduced by Brogniart, may be regarded as a type, though excellent for domestic use, is incapable of receiving a brilliant decoration.

