 полная версия
полная версияStudies in the Theory of Descent, Volume II
The subdorsal line extends from immediately behind the second eye-spot to the base of the very short and much curved violet anal horn. With reference to the protective colouring Mr. Trimen writes: – “The difficulty of seeing these large and beautifully-coloured larvæ on the vines is quite surprising; six or more may be well within sight, and yet quite unnoticed. The subdorsal stripe greatly aids in their concealment, as it well represents in its artificial light and shade the leaf-stalks of the vine.” When this larva withdraws its front segments the eye-spots stand out very menacingly; but in spite of this it is greedily eaten by fowls and shrikes (Fiscus Collaris), and Mr. Trimen also found that a tame suricate (Rhyzæna Suricata) and a large monitor lizard (Regenia Albogularis) did not refuse them. The failure of the eye-spots in causing terror in these particular cases cannot be regarded as disproving their utility in all instances. It must always be borne in mind that no protective character can possibly be of service against all foes; natural selection only requires that such characters should be advantageous with respect to the majority of the enemies of any species, and further experiments with this caterpillar may show that in the case of smaller foes the eye-spots are effective as a means of causing alarm. The dimorphism of the larva of C. Capensis is of special interest, although we are not yet sufficiently acquainted with the habits of this species to offer a complete explanation. According to Dr. Weismann’s conclusions (p. 297), the dimorphism of the Chærocampa-larvæ is due to a double adaptation, the insects first having acquired the habit of concealing themselves by day, and the dark form having then been produced by the action of natural selection, in order to adapt such varieties to the colour of the soil, whilst others retained the green colour which adapts them to the foliage of their food-plants. In accordance with this, C. Capensis may have a similar habit of concealment, or (should this be found not to be the case) it is possible that this insect at a former period possessed this habit and fed upon some other plant, when it would have become dimorphic in the manner explained, and the existing dimorphism may be a survival of the more ancient dimorphism, the red form (corresponding to the older dark form) having been subsequently modified so as to become also adapted to the new food-plant. Much light would be thrown upon this by studying the ontogeny of the species.
Phytophagic Variability.– A number of observations bearing on the phytophagic variability of the Sphinx-larvæ and other caterpillars have been recorded in a previous note (p. 305), and reference has also been made to the food-plants of Acherontia Atropos in South Africa (note 121, p. 263). I am now enabled to add some further observations on this species, from notes furnished to me by Mr. Roland Trimen, who states that for many years he has noticed that at the Cape this larva varies greatly in the depth and shade of the green ground-colour, the variability being in strict accordance with the colour of the leaves of the particular plant on which the individual feeds. The phenomenon was particularly noticeable in larvæ feeding on Buxia Grandiflora, a shrub in common cultivation in gardens, and of which the foliage is of a very dull pale greyish-green. Another striking instance was noticed in some very fine caterpillars feeding on a large shrubby Solanum, which, excepting the bright yellow bands bordering the dorsal violet bars, were generally dull ochreous-yellow, like the leaves and stalks of the Solanum. On plants with bright green or deep green leaves, the colour of the larvæ is almost in exact agreement. Mr. Trimen adds: – “These remarks apply principally to the underside and pro-legs and lower lateral regions, the dorsal colours of violet and yellow varying but little. The protection afforded is very considerable, as the larvæ almost always cling to the lower side of the twigs of their food-plants, so that their uniformly-coloured under-surface is upwards, and turned towards the light, and their variegated upper surface turned downwards.”
These observations are of the highest importance, not only as adding another instance to the recorded cases of phytophagic variation, but likewise as showing that with this variability a protective habit has been acquired. It is to be hoped that such a promising field for experimental investigation as is offered by this and analogous cases will not long remain unexplored. In attacking the problem two chief questions have in the first place to be settled: (1) Is the variability truly phytophagic, i. e. are the colour variations actually brought about by the chemico-physiological action of the food-plant? and (2) Are the larvæ at any period of growth susceptible to the action of phytophagic influences? The first question could be decided by feeding larvæ from the same batch of eggs on different food-plants from the period of their hatching. The second question could be settled by changing the food-plants of a series of selected specimens at various stages of growth, and observing whether any change of colour was produced. In accordance with the principles advocated in a previous note (p. 305), it is conceivable à priori that phytophagic variability may occur by direct chemico-physiological action, quite irrespective of any of the changes of colour being of protective use. In the case of brightly-coloured distasteful species phytophagic variability might thus have full play, but in the case of protectively-coloured edible species, phytophagic variability would be under the control of natural selection. These considerations raise a question of the greatest theoretical interest in connection with this phenomenon. If phytophagic variability can have full play uncontrolled by natural selection in brightly-coloured caterpillars, ought not this phenomenon to be of more common occurrence in such species than in those protectively coloured? Although our knowledge of this subject is still very imperfect, as a matter of fact brightly coloured larvæ, so far as I have been able to ascertain, do not appear to be susceptible of phytophagic influences. But this apparent contradiction, instead of opposing actually confirms the foregoing views, as will appear on further consideration. The colours of protected species are as a whole much inferior in brilliancy to those of inedible species, so that any phytophagic effect would be more perceptible in the former than in the latter, in which the highest possible standard of brilliancy appears in most cases to have been attained. Now phytophagic variations of colour appear to be of but small amount, or, in other words, such variations fluctuate within comparatively restricted limits, and as the cases at present known are mostly adaptive it is legitimate to conclude that they have been produced and brought to their present standard by natural selection, i. e. that they have arisen from phytophagic influences as a cause of variability. The initial stages of phytophagic variations must therefore have been still less perceptible than the now perfected final results; and this leads to the conclusion that minute variations of this character were of sufficient importance to protectively-coloured species to be taken advantage of by natural selection. But minute variations in a dull-coloured larva would, as previously pointed out, produce a comparatively much greater effect than such variations in a brilliantly-coloured species; and as protection is required by the former, the initial phytophagic effects would be accumulated, and the power of adaptability conferred by the continued action of natural selection, whilst in vividly-coloured species where no power of adaptability is required this cause of variation would not only produce a result which, as compared with its effects upon dull species, may be regarded as a “vanishing quantity,” but this result would be too insignificant to be taken advantage of by natural selection, which is in these cases dealing only with large “quantities,” and striving to make the caterpillars as brilliant as possible. The fact that vividly-coloured distasteful larvæ do not show phytophagic variation is to my mind explained proximately by these considerations; the ultimate cause of phytophagic variability regarded as a chemico-physiological action requires further investigation.
Sexual Variation in Larvæ.– Since most of the markings of caterpillars can be explained by the two factors of adaptation and inheritance, or, in other words, by their present and past relations to the environment, and since sexual selection can have played no direct part in producing these colours and markings, I feel bound to record here some few observations on the sexual differences in larvæ in addition to the cases of Anapæa and Orgyia already recorded (note i., p. 308) and of Lophostethus Dumolinii (p. 527).
Mr. C. V. Riley states49 with reference to the larva of Thyreus Abboti that the ground-colour appears to depend upon the sex, Dr. Morris having described the insect as “reddish-brown with numerous patches of light green,” and having expressly stated that “the female is of a uniform reddish-brown with an interrupted dark-brown dorsal line and transverse striæ.” Mr. W. D. Gooch, who has reared the South African butterflies Nymphalis Cithæron and N. Brutus from their larvæ, states50 that these “differed sexually in both instances.” Of Brutus only a few were bred, but of Cithæron many. “The sexual difference of the latter was that the females had a large dorsal sub-cordate cream mark, which was wanting, or only shown by a dot, in the males, and the colour was more vivid in the edgings to the frontal horns.”
Although such cases appear to be at present inexplicable, they are of interest as examples of those “residual phenomena” which, as is well known, have in many branches of science so often served as important starting-points for new discoveries and generalizations.51
APPENDIX II
The following paper by Dr. Fritz Müller52 forms the third of a series of communications on Brazilian butterflies published in “Kosmos,” and as it bears upon the investigations made known in the third essay of the present work, I will here give a translation, by permission of the publisher, Herr Karl Alberts.
“Acræa and the Maracujá Butterflies as Larvæ, Pupæ, and Imagines“In a thoughtful essay on ‘Phyletic Parallelism in Metamorphic Species,’ Weismann has shown that in the case of Lepidoptera the developmental stages of larva, pupa, and imago vary independently, and that a change occurring in one stage is without influence upon the preceding and succeeding stages, so that the course which has been followed by the individual stages in their developmental history has not been in all cases identical. This want of agreement may manifest itself both by unequal divergence of form-relationship, and by unequal group formation. With respect to unequal form-divergence the caterpillars are sometimes more closely related in form than their imagines, and at other times the reverse is the case. With respect to unequal group formation again, two cases are possible; the larvæ and imagines may form groups of unequal value, the one stage forming higher or lower groups than the other, or they may form groups of unequal size, i. e., groups which do not coincide but which overlap. Form-relationship and blood-relationship do not therefore always agree; the resemblances among the caterpillars would lead to a quite different arrangement to that resulting from the resemblances among the imagines, and it is probable that neither of these arrangements would correspond with the actual relationships.
“Starting from this fact, which he establishes by numerous examples, Weismann proceeds to show most convincingly that an innate power of development or of transformation, such as has been assumed under various names by many adherents of the development theory, has no existence, but that every modification and advancement in species has been called forth by external influences.
“A most beautiful illustration of the want of ‘phyletic parallelism,’ as Weismann designates the different form-relationships of the larvæ, pupæ, and imagines, is furnished by the five genera Acræa, Heliconius, Eueides, Colænis, and Dione (= Agraulis). This instance seems to me to be of especial value, because it offers the rare case of pupæ showing greater differences than the larvæ and imagines.
“The species of which I observed the larvæ and pupæ are Acræa Thalia and Alalia, Heliconius Eucrate, Eueides Isabella, Colænis Dido and Julia, Dione Vanillæ and Juno; besides these I noticed the pupa of Eueides Aliphera.
“The following remarks apply only to these species, although we may suppose with great probability that the whole of the congeneric forms – excepting perhaps the widely ranging species of Acræa– would display similar characters to their Brazilian representatives.
“The imagines of the five genera mentioned form two sharply defined families, the Acræidæ and the butterflies of the Maracujá group.53 The latter comprises the three genera Heliconius, Eueides, and Colænis, which differ only in very unimportant characters; Eueides is distinguished from Heliconius by its shorter antennæ, and Colænis differs from Eueides in having the discoidal cell of the hind-wings open. The genus Dione is further removed by the different structure of the legs, and the silvery spots on the underside of the wings. Certain species resemble those of other genera in a most striking manner, and much more closely both in colour and marking, and even in the form of their wings, than they do their own congeners. This is the case with Acræa Thalia and Eueides Pavana, with Heliconius Eucrate and Eueides Isabella, and with Eueides Aliphera and Colænis Julia, which are deceptively alike, and the last two are connected with Dione Juno, at least by the upper side of the wings. The difficulty of judging of the relationships of the single species is thus much aggravated; it cannot be said how much of this resemblance is to be attributed to blood-relationship, and how much to deceptive imitation.
“As larvæ all the Brazilian species must be placed in one genus, as they agree exactly in the number and arrangement of their spines (4 spines, not in a transverse row, on segments 2 and 3; 6 spines, in a transverse row, on segments 4–11; 4 spines, not in a transverse row, on the last (12th) segment). They differ from one another much less in this respect than do the German species of Vanessa, such, for instance, as V. Io or Antiopa from V. Polychloros, Urticæ, and Atalanta.54 The larvæ of Acræa Thalia are certainly without the two spines on the head which the others possess, and, on the other hand, they have a well-developed pair of spines on the first segment, which, in most of the other species, are completely absent; but this does not justify their separation, since the head spines of Heliconius, Eueides, and Colænis Dido, which are of a considerable length, are shorter than those of the next segment in Colænis Julia, and Dione Vanillæ, and in Dione Juno they dwindle down to two minute points, this last species also bearing a short pair on the first segment. The larva of Dione Juno is thus as closely related to that of Acræa Thalia as it is to that of its congener Dione Vanillæ.
“If it were desired to form two distinct larval groups this could not be effected on the basis of their differences in form, but could only be based on their food-plants. The larvæ of Heliconius, Eueides, Colænis, and Dione live on species of Maracujá (Passiflora); those of Acræa Thalia and Alalia on Compositæ (Mikania and Veronia). These larval groups would agree with those founded on the form-relationships of the imagines, but unlike the imaginal groups, which can be formed into families, they would scarcely possess a generic value.
“If we arrange the single species of caterpillars according to their resemblances, this arrangement does not agree with that based on the resemblances of the imagines, even if we disregard the different values of the groups. The result is somewhat as follows: —
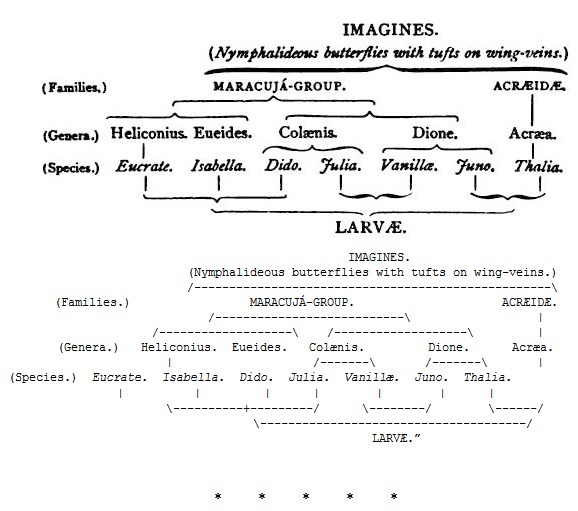
[Here follow the remarks on the habits of the larvæ in connection with their colours, &c., which have already been quoted in illustration of the use of the spiny protection (note 133, p. 293). From these facts the author draws the conclusion that the form-relationships of the caterpillars depend rather upon their mode of life than upon their blood-relationships, assuming the latter to be correctly expressed by the arrangement of the imagines at present adopted.]
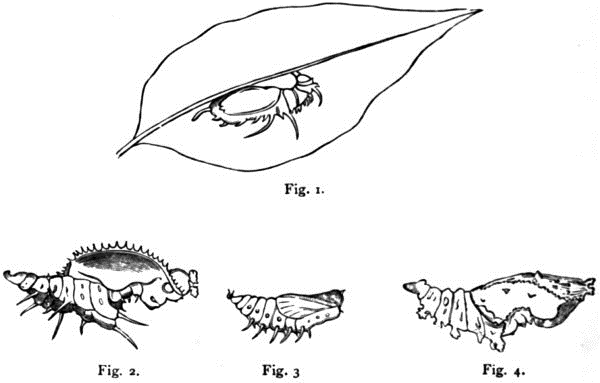
Figs. 1–4. Pupæ of Acræa Thalia; Heliconius Eucrate; Eueides Isabella, and Colænis Dido; life size.
“A glance at the above figures of the pupæ of Heliconius Eucrate (Fig. 2), Eueides Isabella (Fig. 3), and Colænis Dido (Fig. 4), will show how great are the differences between these pupæ as compared with the close form-relationship of all the Maracujá butterflies, and with the no less close resemblance of their larvæ. A family which comprised three such dissimilar pupæ would also be capable of including that of Acræa Thalia (Fig. 1).
“The pupa of this last species has nothing peculiar in its general appearance, but possesses the ordinary pupal form; it is tolerably rounded, without any great elevations or depressions; a minute pointed projection is situated on the head over each eye-cover, and a similar process projects from the roots of the wings. Its distinguishing characters are five pairs of spines on the back of the abdominal segments. These spines are found also in Acræa Alalia, but appear to be absent in other species, e. g. in the Indian A. Violæ. Last summer, among some batches of Thalia larvæ – each batch being the progeny from one lot of eggs – I found certain individuals which differed from the others in having much shorter spines, and these changed into pupæ in which the five pairs of spines were proportionally shorter than usual, thus being an exception to the rule that changes in one stage of development are without influence on the other stages. I may remark, by the way, that this law, enunciated by Weismann, can only be applied to imagines and pupæ with certain restrictions. The skin of the pupa forms a sheath or cover for the eyes, antennæ, trunk, legs, and wings of the imago, and if these parts undergo any considerable modification in the latter, corresponding changes must appear in the pupa. This is shown, for instance, by many ‘Skippers’ (Hesperidæ), in which the extraordinarily long trunk necessitates a sheath of a corresponding length. The colour of the pupa of Acræa Thalia is whitish, the wing-veins with some other markings and the spines are black; metallic spots are absent.
“In the pupa of Heliconius Eucrate the laterally compressed region of the wings is raised into a large projection, the antennal sheaths lying on the edges of the wings are serrated and beset with short pointed spines; instead of the minute projections of Acræa Thalia, the head bears two large humped processes; the body is raised on each side into a foliaceous border carrying five spines of different lengths, the foremost pair, directed towards the head, being the longest. The pupa is brown, and ornamented with four pairs of brilliant metallic spots, one pair close behind the antennæ, and three pairs, almost coalescent, on the back before the longest pair of spines. A short spine projects from the middle of each of the latter somewhat arched metallic patches.
“In the pupa of Colænis Dido (which resembles that of Colænis Julia, and to which may be added those of Dione Vanillæ and Juno) the spines are absent, the wing region is but moderately arched, and the antennæ marked only by small elevations; instead of the leaf-like border, there are on each side of the back five knotty or humped processes. The metallic spots are similar in number and position to those of Heliconius Eucrate; those on the back have a wart-like process in the middle, instead of a spine.
“The pupæ of Heliconius and Colænis when moving their posterior segments rapidly, as they do whenever they are disturbed, produce a very perceptible hissing noise by the friction of these segments, this sound, which is especially noticeable in the case of Heliconius Eucrate, perhaps serving to terrify small foes. (So loud is the sound produced in this manner by the pupæ of Epicalia Numilia, that my children have named them ‘Schreipuppen.’)
“The pupæ of Heliconius and Colænis thus differ to a much greater extent than the imagines or larvæ, and the same holds good for Eueides in a much higher degree as compared with its above-mentioned allies. The larvæ of Eueides have no distinctive characters, and even the generic rank of the imagines is doubtful; as pupæ, on the other hand, they are far removed (even by their mode of suspension) not only from the remainder of the Maracujá group and from the whole of the great Nymphalideous group (Danainæ, Satyrinæ, Elymniinæ, Brassolinæ, Morphinæ, Acræinæ and Nymphalinæ), but from almost all other butterflies. The larva pupates on the underside of a leaf; the pupa is fastened by the tail, but does not hang down like the pupæ of the other Nymphalidæ, – its last segments are so curved that the breast of the chrysalis is in contact with the underside of the leaf. I am not acquainted with any other pupa among those not suspended by a girdle which assumes such a position. Something similar occurs, however, in the pupa of Stalachtis, which is without a girdle, and according to Bates, is ‘kept in an inclined position by the fastening of the tail.’ By this peculiarity Bates distinguishes the Stalachtinæ from the Libytheæ with pupæ ‘freely suspended by the tail.’
“Besides through this peculiar position of the body, the pupa of Eueides Isabella is distinguished by short hooked and long narrow sabre-like pairs of processes on the back and head. Its colour is whitish, yellowish, or sordid yellowish-grey; in the last variety both the four long dorsal processes and the surrounding portions, as well as the points of the other processes, remain white or yellowish. The pupa Eueides Aliphera is very similar, only all the processes are somewhat shorter, the four longest (dorsal) and some other markings being black.
“Now if, as Weismann has attempted to show for larvæ and imagines, the form-divergence always ‘corresponds exactly with the divergence in the mode of life,’ the question arises as to what difference in the conditions of life has brought about such a considerable form-divergence between the pupæ of such closely allied species as the Maracujá butterflies. In pupæ which do not eat or drink, and which have neither to seek in courtship nor to care for progeny, it is only protection from foes that can concern us. But in the pupæ of nearly allied species of which the larvæ feed on kindred plants in the same districts at the same periods of the year, can the enemies be so different as to produce such a considerable divergence in form? One might answer this question in the negative with some confidence, and affirm that in this case the difference in the pupæ does not result from the ‘divergence in the mode of life,’ or from the difference in the external conditions, but is accidental, i. e. a consequence of some fortunate variation induced by some external cause, which variation afforded protection against common foes – to one species in one way, and to the other species in some other way; this course, once entered upon, having been urged on by natural selection, until at length the wide divergence now shown is attained. How in the case of any of the species the peculiarity in colour or form can actually serve as a protection, I must confess myself at fault in answering. Only in the case of the pupa of Eueides Isabella will I venture to offer a supposition. That it is not green like other pupæ which suspend themselves among foliage (Siderone, Epicalia, Callidryas, &c.), but contrasts more or less brightly with the dark green of the leaves, precludes the idea of concealment; on the other hand its colour is too dull to serve as a conspicuous sign of distastefulness. In either case the meaning of the wonderful processes of the pupa would remain unexplained.




