 полная версия
полная версияFirst virtual Bilateral Conference on Functional Materials (BiC-FM)
Green synthesis of reduced graphene oxide for biomedical applications
Dharshini Perumal2, Muhd Amir Faiz Muhd Shaifuddin.2, Emmellie Laura Albert2, Che Azurahanim Che Abdullah 1,2* and Mas Jaffri Masaruddin3
1-Institute of Bioscience, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
2-Biophysics Lab, Department of Physics, Faculty of Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
3-Department of Cell and Molecular Biology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
shinijessy@gmail.com
Graphene is the thinnest and lightest material with prominent properties. This offers an enormous potential for application in various sectors. The synthesis of high-quality graphene in an eco-friendly manner remains a major challenge. Reliable, economical, facile, and chemical reduction method is the option for the preparation of reduced graphene oxide (rGO). This method is considered to be eminent for mass production and offers safety to the environment and human health. Green technology opens new windows in the synthesis of green graphene oxide (GO) reduction using plant extracts[1]. Plant extracts are captivating alternative to the noxious chemical reducing agents. They are readily available, renewable, economical and environmentally friendly in nature. The present study focuses on the synthesis of rGO using extracts from leaves of Elaeis guineensis. Herein we present the preliminary data on structural and optical properties. Based on the characterization data by FTIR, XRD, UV and TEM revealed that we successfully synthesized green rGO [2,3,4]. The potential application of the prepared rGO will be on biomedical applications.
Acknowledgement.This work was partially supported by Fundamental Research Grant Scheme (FRGS), grant number 5524949.
References:
[1] Fahiminia et al., “Phytosynthesis of Cu/rGO using Euphorbia cheiradenia Boiss extract and study of its ability in the reduction of organic dyes and 4-nitrophenol in aqueous medium”, IET Nanobiotechnology, vol. 13, pp. 202–213, 2019
[2] Veisi et al., “In situ biogenic synthesis of Pd nanoparticles over reduced grahene oxide by using a plant extract (Thymbra spicata) and its catalytic evaluation towards cyanation of aryl halides”, Material Science & Engineering C, vol. 104, pp. 109919, 2019
[3] Moosa A.A, Jaafar J. N., “Green Reduction of Graphene Oxide Using Tea Leaves Extract with Applications to Lead Ions Removal from Water”, Nanoscience and Nanotechnology, vol. 7, pp. 38–47, 2017
[4] Medha G., Sharmila C., and Anil G.,”Green Synthesis and Characterization of Nanocrystalline Graphene Oxide”, Int. Res. J. of Science & Engineering, Special Issue A1, pp. 29–34, 2017
Optical properties of carbon nanodots obtained from the Kuzbass basin coals
Prazyan T.L., Dyagilev D.V.
Kemerovo State University, Kemerovo, Russian Federation
Prazyan.tigran@yandex.ru
Carbon nanodots (C-dots) were first obtained in 2004 [1] in the process of electrophoretic purification of carbon nanotubes, and since then the methods of synthesis and control of the functional properties of such structures have been constantly improving. The search for facile and inexpensive methods of C-dots' producing has led researchers to the idea of using coal as a cheap and widely available raw material. One of the first successful attempts of C-dots' fabrication from anthracite and bituminous coal was reported in 2013 [2].
In this work, we employed the coal samples from the Kuznetsk Basin (Anthracite, Bituminous coals), which is the largest source of coal in Russia. In order to effectively extract the C-dots, we have developed a method consisting of the following stages: wet grinding of coal in a planetary ball mill, peroxidation with ultrasonic activation, stabilization and sedimentation of the suspension, evaporation and drying.
It is found that the yield of C-dots for various grades of coal ranges from 1 to 20 %. The absorption spectra were used to estimate the band gap of the emitting particles, which was in the range of 2.3–3.8 eV. Upon excitation with light with a wavelength of 210 nm, the particles exhibited luminescent emission at the wavelengths of 280–500 nm with the strong peaks at 300 and 460 nm. The anthracite derived C-dots exhibited the most pronounced emission at 460 nm, which is possibly due to the presence of heavy metal impurities. Their presence in the form of the inner structural components or adsorbed species is known to strongly affect the optical properties of quantum-sized particles [3].
Acknowledgements.This work was supported by the Ministry of Science and Higher Education of the Russian Federation (project no. FZSR-2020-0007 in the framework of the state assignment no. 075-03-2020-097/1).
References:
[1] X. Xu, R. Ray, Y. Gu, et al., J. Am. Chem. Soc., 126, 12736 (2004)
[2] R. Ye, C. Xiang, J. Lin, et al., Nat. Commun., 4, 2943 (2013)
[3] F. Liu, M.‐H. Jang, H.D. Ha, et al., Adv. Mater., 25, 3657 (2013)
Synthesis of core shell Nano magnets with size tailoring by aerosol CVD
Javier A. Ramirez B.1, Dmitry V. Krasnikov1 Alena A.Alekseeva1, Anastasia E. Goldt1, and Albert G. Nasibulin1,2
1 Skolkovo institute of Science and technology, Centre for Photonics and Quantum Materials, Laboratory of nanomaterials, Moscow, 121205, Russian Federation.
2 Aalto University, Kemistintie 1C, 00076 Aalto, Finland.
javier.ramirez@skoltech.ru
Interest in research of core shell nanoparticles is arising due to the unique, novel properties of these structures. Aerosol synthesis presents advantages compared with other methods, which involve many steps, post processing and are slow, and usually low yield. Scalability is very important for readily useful materials, where bulk amounts with restricted structural parameters are desired.
We propose and demonstrate the results of an innovative, single step, fast, continuous, environmentally friendly, industrially scalable Aerosol Chemical Vapour Deposition, inspired by [1] and based on the setup used in [2] to synthesize nanoparticles with mean size of 50 nm. by using organometallic compounds such as Metallocenes and hydrocarbons (C2H4) as precursors. Morphological properties were observed, size and crystal characteristics were altered by varying reactor conditions, achieving the production of carbon containing nanoparticles in a laminar flow vertical reactor.
Aerosol synthesis of particles was carried out successfully, at atmospheric pressure; concentration of reactants was changed by adjusting saturator temperature. Transmission electron micrographs (Fig. 2) of different particles were taken, XRD analysis of bulk samples was carried out, as well as Dynamic mobility analyser profiles (Fig. 1).

Figure 1. Dynamic mobility analyser profile for Different process temperatures.
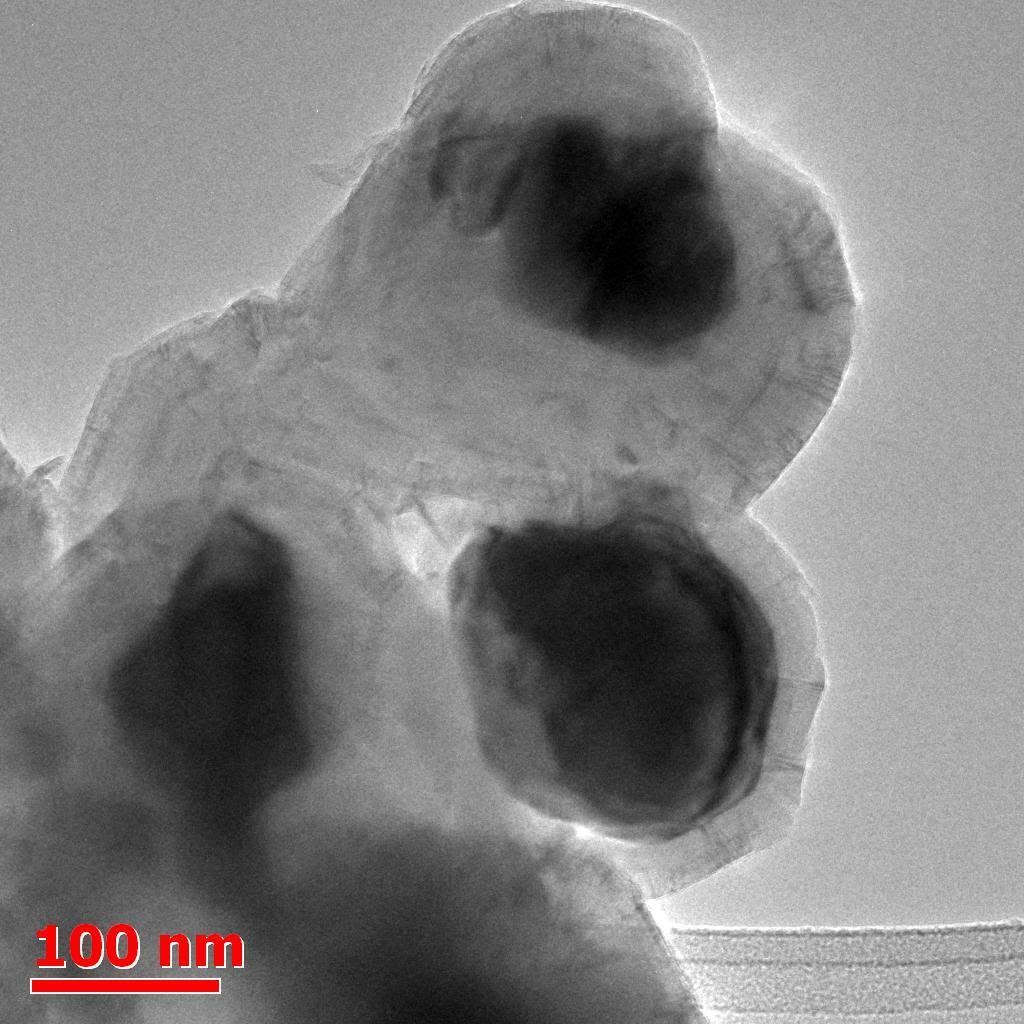
Figure 2. Micrograph of synthesized. Nano magnets.
Acknowledgement.This work was supported by the Skoltech Biomedical Initiative, project Assignment № 2017-7/SBI.
References:
[1] Nasibulin, A. G., Ahonen, P. P., Richard, O., Kauppinen, E. I., and Altman, I. S., Copper and Copper Oxide Nanoparticle Formation by Chemical Vapor Nucleation From Copper (II) Acetylacetonate, Journal of Nanoparticle Research 3, 383–398 (2001).
[2] Kim, D., Vasilieva, E. S., Nasibulin, A. G., Lee, D. W., Tolochko, O. V., & Kim, B. K. (2007). Aerosol synthesis and growth mechanism of magnetic iron nanoparticles. Materials Science Forum. https://doi.org/10.4028/0-87849-419-7.9.
Polyacrylic Acid Modified Cerium Oxide Nanoparticles for Non-Enzymatic H2O2 Sensor
Gurdeep Rattua, Nishtha Khansilia and P.Murali Krishnaa *;
aDepartment of Basic and Applied Science, National Institute of Food Technology Entrepreneurship and Management (NIFTEM) Kundli, Haryana 131028, India
mkprayaga@yahoo.co.in
Background: Cerium oxide nanoparticles (nanoceria) are efficient free-radical scavengers due to their dual valence state and thus exhibit optical and catalytic properties. This research revealed the development of fluorescence hydrogen peroxide nanosensor based on the peroxidase-like activity of polyacrylic acid stabilized nanoceria (PAA-CeO2 Nps). Method: PAA-CeO2 Nps were synthesized by simple cross-linking reaction at a low temperature and characterized by XRD, SEM, Zeta potential, TGA, FT-IR and UV–VIS spectroscopic analysis. H2O2 sensing was performed by a spectrophotometer.The XRD diffraction patterns confirmed the polycrystalline nature and SEM micrograph showed nanoparticles having hexagonal symmetry and crystallite size of 32 nm. UV–VIS measurements revealed a well defined absorbance peak around 315 nm and an optical band-gap of 3.17 eV by Tauc plots. As synthesized PAA-CeO2 Nps effectively catalysed the decomposition of hydrogen peroxide (H2O2) into hydroxyl radicals. Then terephthalic acid was oxidized by hydroxyl radical to form a highly fluorescent product. Under optimized conditions, the linear range for determination of hydrogen peroxide was 0.01 – 0.2 mM with a limit of detection (LOD) of 1.2 µM. The proposed method is ideally suited for the sensing of H2O2 at a low cost and this detection system enabled the sensing of analytes (sugars), which can enzymatically generate hydrogen peroxide.
Composites Nano-Titania Graphite for Photocatalytic and Antibacterial Activities
Natrah Shafiqah Rosli1,2,3 , Che Azurahanim Che Abdullah1,2, Roshasnorlyza Hazan3
1Institute of Advance Technology (ITMA), University Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia.
2Department of Physics,Faculty of Science, University Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia.
3Malaysian Nuclear Agency, Bangi, 43000 Kajang, Selangor, Malaysia.
natrahshafiqahrosli@gmail.com
Nano-Titania or Titanium dioxide (TiO2) nanoparticles is a well-known photocatalyst that widely used for environmental cleanup due to its ability to decompose organic pollutant and kill bacteria [1]. Although TiO2 nanoparticles have the advantages to be used in the environmental concern, their functionality depends mainly on both the precursor and the method used [2]. Typically, the synthesized TiO2 nanoparticles involved expensive methods and the usage of hazardous chemical reagents. In this research, the low cost, easy to prepare, and environmental of synthesized TiO2 nanoparticles were utilized. TiO2 nanoparticles in anatase phase were successfully prepared via the alkaline fusion method using synthetic rutile as a precursor. The synthetic rutile used in this project was derived from natural Malaysian Ilmenite’s waste to produce a low cost of TiO2 nanoparticles via the environmentally friendly process and relative simplicity for a large scale. In attempts to improve the performance of the synthesized TiO2 nanoparticles, some modifications were carried out to enhance their photocatalytic properties. The synthesized TiO2 nanoparticles were incorporated with graphite as a non-metal material to produce TiO2/G nanocomposites. The incorporation of non-metal material did not change the phase of TiO2 nanoparticles but have a profound impact on the morphology and optical properties were affected as revealed from characterization by using XRF, XRD, TEM, PSA, FTIR, and UV–Vis analysis. The efficiency of synthesized samples as for potential photocatalytic application was examined by the capability in the degradation of methylene orange (MO) as a model of water pollutant under UV-irradiation. TiO2/G nanocomposites showed the best performance where 10 ppm MO degrading up to 93 % for 5 hours. Meanwhile, the modified samples were also tested by using Gram-negative bacterial strain Escherichia coli (E-coli) for the antibacterial activities test. The antibacterial activities were successfully evaluated based on the diameters of clear inhibition zone surrounding paper disk after 24 hours. From the results obtained, TiO2/G nanocomposites have proven to be efficient antibacterial material to inhibit E-coli. The results obtained revealed and established that TiO2/G nanocomposites have excellent performance for the degradation of MO in photocatalytic tests as well as having the ability to inhibit E-coli in antibacterial activities.
Acknowledgement.We would like to express our deepest gratitude to the supporting staff of Materials Technology research Group (MTEC), Malaysian Nuclear Agency for funding this project as well as NANOTEDD members in the Biophysics Lab, Department of Physics, UPM.
References:
[1] Akhtar, S., Ali, I., & Tauseef, S. (2016). Synthesis, Characterization and Antibacterial Activity of Titanium Dioxide (TiO2) Nanoparticles. Fuuast Journal of Biology, 6(2), 141–147.
[2] Chong, M. N., Jin, B., Chow, C. W., & Saint, C. (2010). Recent developments in photocatalytic water treatment technology: a review. Water research, 44(10), 2997–3027.
High-yield synthesis of single-walled carbon nanotube films for targeted applications
Shaikhulova A.R.1, Khabushev E.M.1,2, Krasnikov D.V.1, Nasibulin A.G.1,2
1 – Skolkovo Institute of Science and Technology, Nobel 3, 121205, Moscow, Russia
2 – Aalto University School of Chemical Engineering, Kemistintie 1, 02015, Espoo, Finland
alisa.shaikhulova@skoltech.ru
Single-walled carbon nanotubes (SWCNTs) possess striking structural and electronic properties, which has drawn significant attention of scientists and researchers in the past 25 years [1]. This material can be used in a broad variety of applications like field emitters, supercapacitors, sensors, etc. [2]. One of the main challenges preventing common SWCNT implementation is cost-effective large-scale synthesis of high-quality nanotubes. Aerosol chemical vapor deposition (CVD) method is believed to be one of the most promising techniques for industrial nanotube production, which is easy to scale-up, however, lacks productivity [3].
We have tackled this problem by the development of cost-effective aerosol CVD method for SWCNT synthesis using ethylene as a carbon source, ferrocene as a catalyst precursor, and carbon dioxide as a promoter with a focus on transparent and conductive applications. The influence of various factors on SWCNT film production was examined by a complex set of methods (four-probe resistance measurements, Raman and UV-vis-nIR spectroscopy, SEM and TEM). Investigating synthesis conditions (temperature, catalyst precursor and reactant partial pressure), we found ethylene and carbon dioxide concentrations of 0.4 vol. % to correspond to the highest yield and to the lowest equivalent sheet resistance. At the same time, the variation ferrocene concentration from 0.05 Pa to 0.18 Pa revealed a trade-off between yield and conductivity. Obtained results will guide to further development of scalable and relatively low-cost process for SWCNT synthesis, with the most voluminously produced hydrocarbon and environmentally friendly growth process.
Acknowledgement.This work was supported by Russian Science Foundation project No. 20-73-10256.
References:
[1]. T. W. Odom, J.-L. Huang, P. Kim, C. M. Lieber. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature, 391(6662), 62–64 (1998).
[2]. M. F. L. De Volder, S. H. Tawfick, R. H. Baughman, A. J. Hart. Carbon nanotubes: present and future commercial applications. Science, 339(6119), 535–539 (2013).
[3]. M. Kumar, Y. Ando. Chemical Vapor Deposition of Carbon Nanotubes: A Review on Growth Mechanism and Mass Production. Journal of Nanoscience and Nanotechnology, 10(6), 3739–3758 (2010).
Protective spinel coating for Li1.17Ni0.17Mn0.50Co0.17O2 cathode for Li-ion batteries through single-source precursor approach
A. Shevtsov 1, H. Han 2,3, E.V. Dikarev3 and A.M. Abakumov1
1-Skolkovo Institute of Science and Technology, Moscow, 143026 Russia
2-Department of Materials Science and Engineering, Cornell University, New York 14850, USA
3-State University of New York at Albany, 1400 Washington Ave, Albany, NY 12222 USA
andrey.shevtsov@skolkovotech.ru
The layered oxide Li1.16Ni0.16Mn0.5Co0.16O2 (Li-rich NMC) may exhibit a reversible capacity of 300mAh/g at C/20 rate for several cycles with further capacity fade. Its commercialization is impossible due to the degradation, which is attributed to the interaction of the material with the electrolyte at high voltages. The particle surface coating is an approach towards the physical separation of the cathode and electrolyte to diminish the capacity and voltage fade.
The employment of multi-source precursors to prepare specific functional materials may result in an inhomogeneous chemical distribution [1]. A surface-sensitive application, such as coatings, may result in the lack of the desired electrochemical properties. Thus, there is an interest in exploring new flexible synthetic pathways that yield “molecular pure” materials. Amongst the possible solutions: the single-source precursor approach – the usage of molecular precursors, which upon decomposition form the desired crystal phase [2].
The molecular precursor to the manganese spinel [3], LiMn2(thd)5 (thd = 2,2,6,6‐tetramethyl‐3,5‐heptanedionate), was used as a parent molecule to further design the synthesis of the substituted manganese spinel, LiMn1.5M0.5O4. Thereafter, the respective complex LiMnCo(thd) was successfully isolated and upon decomposition yielded the spinel oxide, LiMn1.5Co0.5O4 [4]. These molecular precursors were then dispersed on the surface of the layered oxide Li1.16Ni0.16Mn0.5Co0.16O2 (Li-rich NMC) and upon decomposition at 400oC a core-shell cathode material was synthesized. The phase composition of the samples was characterized using powder X-ray diffraction coupled with a high-resolution TEM imagery. The HRTEM imagery of the Li-rich NMC particles shows an external layer 10–20 nm thick. The diffractions confirm the co-existence of the Li-rich NMC (C2/m) and spinel structures (Fm-3m). The sample of Li-rich NMC coated with ~12 wt.% of spinel and annealed at 400 ºC reveals much better capacity retention of 71 % compared to 50 % for the pristine material, as well as better structural stability over the first 25 cycles.
Acknowledgement.This research was supported by the CRDF Global (grant FSCX-16-62133-0).
References:
[1] P.T. Barton et al, M. J. Chem. Eur., J., 19, 14521‐14531 (2013)
[2] H. Han et al, Dalton Trans., 46, 5644‐5649 (2017)
[3] A. Navulla et al, J. Am. Chem. Soc., 134, 5762‐5765 (2012)
[4] H. Haixiang et al, Chem. Sci., 10, 524–534 (2019)
Nitrogen – doped porous carbon obtained by precipitation of acetonitrile vapors on template C–CaO nanoparticles for electrochemical applications
Shlyakhova E.V.1, Fedoseeva Y.V.1, Nischakova A.D.1, Vorfolomeeva A.A.1, Bulusheva L.G.1, Okotrub A.V.1
1 – Nikolaev Institute of Inorganic Chemistry, Novosibirsk, Russia
shlyakhovaev@niic.sbras.ru
Porous nitrogen-containing carbon nanomaterials are promising electrode materials for new generation electrochemical current sources due to developed pore texture, high electronic conductivity and high thermal stability. The synthesis of nitrogen-containing carbon nanomaterials was carried out in several stages: thermal decomposition of organic calcium salts of tartaric, glutaric, and adipic acids at 750 °C, subsequent CVD carbon deposition using acetonitrile as a source, and removal of template particles by treatment with an HCl solution. It has been established that as a result of the thermolysis of calcium salts, composite template particles are mainly consisting of CaO and carbon. The deposition of acetonitrile vapor on their surface leads to the formation of nitrogen-containing graphite-like layers containing 3–5 at.% nitrogen. Removal of template particles from the material leads to the formation of nanopores in the carbon material. The pore size of carbon materials varies from 3 to 30 nm, the gravimetric surface area is from 188 to 832 m2/g, and the specific pore volume is from – 0.4 to 1.6 cm3/g. The influence of the nature of calcium salts of carboxylic acids, which were the sources of template particles, on the structure and electrochemical characteristics of nitrogen-containing carbon nanomaterials was investigated.
Acknowledgement.This work was supported by the Russian Science Foundation, grant 19-73-10068.
N-doped graphene nanoflakes for catalysis and tribology
Stolbov D.N.1,2, Chernyak S.A.1, Usol’tseva N.V.2 Savilov S.V.1, Parfenov A.S.3
1 – Department of Chemistry, Lomonosov Moscow State University, Moscow, Russia
2 – Ivanovo State University, Nanomaterials Research Institute, Ivanovo, Russia
3 – Ivanovo State Academy of Medicine, Ivanovo, Russia
stolbovdn@gmail.com
High demand for carbon nanomaterials (CNM) can be explained by the variety of their chemical and physical properties, as well as high potential of structure modification and possibility of introduction into various matrices to obtain various composite materials [1]. Changes in electronic structure and the formation of different surface defects can be achieved by the partial replacement of carbon atoms by heteroatoms, in particular, nitrogen ones.
In this work, pristine and N-doped graphene nanoflakes (GNF and N-GNF) were synthesized by template pyrolysis and studied by set of physicochemical methods. To study the effect of doping, the samples were studied as cobalt catalyst supports for the Fischer-Tropsch process, as well as additives for industrially produced lubricants.
It was found that the introduction of nitrogen atoms increases the dispersion of the deposited metal, thereby increasing the activity of the catalyst. Also, in the model system, the largest decrease in the friction coefficient (up to 42 %) was exhibited by N-GNF at a concentration of 1.5 wt% at high loads.
Acknowledgement.This work was supported by the Russian Foundation for Basic Research (grant no. 18-29-19150_mk) and supported by the Ministry of Education and Science of the Russian Federation in the framework of the state task for Ivanovo State University (Application No FZZM2020-0006)
References:
[1] Savilov S.V., Ivanov A.S., Egorov A.V., Kirikova M.N., Arkhipova E.A., Lunin V.V. Effect of the morphology of structured carbon nanomaterials on their oxidizability. Russ. J. Phys. Chem. A N.2, V.90, 2016, 429-435
Thermal shock as a new approach for the synthesis of porous MoS2
Stolyarova.S.G.1, Kotsun A.A.2, A. V. Okotrub,1 L. G. Bulusheva1
1 – Nikolaev Institute of Inorganic Chemistry SB RAS, Novosibirsk, Russia
2 – Novosibirsk State University, Novosibirsk, Russia
stolyarova@niic.nsc.ru
The development of technology and user needs requires more powerful devices, energy sources, and materials. Metal ion batteries are widely used now and they can be easily modified by changing electrode materials. MoS2 is actively explored as a promising anode material. Theoretical specific capacity of MoS2 provided by the intercalation and conversion reactions is 669 mAh g-1, which is 2 times higher than the corresponding value for graphite. The main disadvantage of bulk MoS2 as an anode material is the short battery life. The MoS2-based material can be stabilized by using a carbon component, but an alternative approach is to obtain nanostructured MoS2. In this case, decreased size and formation of defects and pores contribute to a high capacity and decreased resistance of the material and increased diffusion rate of lithium ions. Traditionally, nanostructured porous sulfides are synthesized by self-assembling in solution or with use of templates.









