
Полная версия
The Fontana History of Chemistry
While still a phlogistonist, Guyton was much exercised by the inconsistent nomenclature of chemists and pharmacists. Unlike botany and zoology, whose terminology had been revised and made more precise earlier in the century by the
TABLE 3.1 The contents of Fourcroy’s Système des connaissances chimiques (1800) arranged by classes of substances.
Class Volume 1. Undecomposed or simple bodies 1 2. Burned bodies; oxides or acids 2 3. Salifiable bases; earths and alkalis 2 4. Salts 3, 4 5. Metals and metal salts containing an excess of acid (although belonging to class 1, these were dealt with separately because of their number and importance) 5, 6 6. Minerals 6 7. Vegetable compounds 7, 8 8. Animal compounds 9, 10Swede, Karl Linnaeus, chemical language remained crude and confusing. In 1782 Guyton made a series of proposals for the systematization of chemical language.
Alchemical and chemical texts written before the end of the eighteenth century can be difficult to read because of the absence of any common chemical language. Greek, Hebrew, Arabic and Latin words are found, there was widespread use of analogy in naming chemicals or in referring to chemical processes, and the same substance might receive a different name according to the place from which it was derived (for example, Aquila coelestis for ammonia; ‘father and mother’ for sulphur and mercury; ‘gestation’ as a metaphor for reaction; ‘butter of antimony’ for deliquescent antimony chloride; and ‘Spanish green’ for copper acetate). Names might also be based upon smell, taste, consistency, crystalline form, colour, properties or uses. Although several of these names have lingered on as ‘trivial’ names (which have even had to be reintroduced in organic chemistry in the twentieth century because systematic names are too long to speak), Lavoisier and his colleagues in 1797 decided to systematize nomenclature by basing it solely upon what was known of a substance’s composition. Since the theory of composition chosen was the oxygen system, Lavoisier’s suggestions were initially resisted by phlogistonists; adoption of the new nomenclature involved a commitment to the new chemistry.
Following the inspiration of Linnaeus, Guyton suggested in 1782 that chemical language should be based upon three principles: substances should have one fixed name; names ought to reflect composition when known (and if unknown, they should be non-committal); and names should generally be chosen from Greek and Latin roots and be euphonious with the French language. In 1787, Guyton, together with Lavoisier, Berthollet and Fourcroy, published the 300-page Méthode de nomenclature chimique, which appeared in English and German translations a year later. One-third of this book consisted of a dictionary, which enabled the reader to identify the new name of a substance from its older one. For example, ‘oil of vitriol’ became ‘sulphuric acid’ and its salts ‘sulphates’ instead of ‘vitriols’; ‘flowers of zinc’ became ‘zinc oxide’.
Perhaps the most significant assumption in the nomenclature was that substances that could not be decomposed were simple (i.e. elements), and that their names should form the basis of the entire nomenclature. Thus the elements oxygen and sulphur would combine to form either sulphurous or sulphuric acids depending on the quantity of oxygen combined. These acids when combined with metallic oxides would form the two groups of salts, sulphites and sulphates. In the case of what later became called hydrochloric acid, Lavoisier assumed that he was dealing with an oxide of an unknown element, murium. Because of some confusion over the differences between hypochlorous and hydrochloric acids, in Lavoisier’s nomenclature hydrochloric acid became muriatic acid and the future chlorine was ‘oxygenated muriatic acid’. The issue of whether the latter contained oxygen at all was to be the subject of fierce debate between Davy, Gay-Lussac and Berzelius during the three decades following Lavoisier’s death.
The French system also included suggestions by Hassenfratz and Adet for ways in which chemicals could be symbolized by geometrical patterns: elements were straight lines at various inclinations, metals were circles, alkalis were triangles. However, such symbols were inconvenient for printers and never became widely established; a more convenient system was to be devised by Berzelius a quarter of a century later.
During the eighteenth century some chemists had turned their minds to quantification and the possible role of mathematics in chemistry. On the whole, most chemists agreed with Macquer that chemistry was insufficiently advanced to be treated mathematically. Although he believed, correctly as it turned out, that the weight of bodies bore some relationship to chemical properties and reactions, the emphasis on affinity suggested that the project was hopeless. Nevertheless, Lavoisier, inspired by the writings of the philosopher, Condillac, believed fervently that algebra was the language to which scientific statements should aspire7:
We think only through the medium of words. Languages are true analytical methods. Algebra, which is adapted to its purpose in every species of expression, in the most simple, most exact, and best manner possible, is at the same time a language and an analytical method. The art of reasoning is nothing more than a language well arranged.
In a paper on the composition of water published in 1785, Lavoisier stressed that his work was based upon repeated measuring and weighing experiments ‘without which neither physics nor chemistry can any longer admit anything whatever’. Again, in another essay analysing the way metals dissolve in acids, Lavoisier used the Hassenfratz – Adet symbols:
In order to show at a glance the results of what happens in the solution of metals, I have constituted formulae of a kind that could at first be taken for algebraic formulae, but which do not have the same object and which do not derive from the same principles; we are still very far from being able to obtain mathematical precision in chemistry and therefore I beg you to consider the formulae that I am going to give you only as simple annotations, the object of which is to ease the workings of the mind.
The important point here was that Lavoisier used symbols to denote both constitution and quantity. Although he did not use an equals sign, he had effectively hit upon the idea of a chemical equation. As we shall see, once Berzelius’ symbols became firmly established in the 1830s, chemists began almost immediately to use equations to represent chemical reactions.
While producing the Méthode de nomenclature chimique with Lavoisier and the others, Guyton was converted to the new chemistry. Because the new language was also the vehicle of anti-phlogiston chemistry, it aroused much opposition. Nevertheless, through translation, it rapidly became and still remains the international language of chemistry.
TABLE 3.2 Lavoisier’s ‘elements’ or ‘simple substances’.
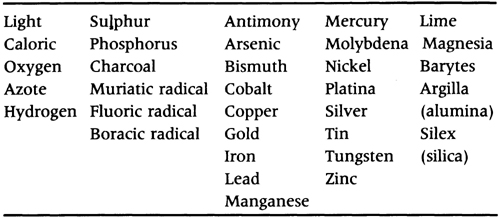
Lavoisier’s final piece of propaganda for the new chemistry was a textbook published in 1789 called Traité élémentaire de chimie (An Elementary Treatise on Chemistry). Together with Fourcroy’s larger text (published in 1801), this became a model for chemical instruction for several decades. In it Lavoisier defined the chemical element pragmatically and operationally as any substance that could not be analysed by chemical means. Such a definition was already a commonplace in mineralogical chemistry and metallurgy, where the analytical definition of simple substances had become the basis of mineralogical classification in the hands of J. H. Pott, A. F. Cronstedt and T. Bergman. It was for this reason that Lavoisier’s list of 33 basic substances bore some resemblance to the headings of the columns in traditional affinity tables. Lavoisier’s list included substances such as barytes, magnesia and silica, which later proved to be compound bodies.
After discussing the oxygen theory in part I of the Traité, he discussed their preparation and properties, their oxides and then their salts formed from acidic and basic oxides in part II. Caloric disengaged from oxygen explained the heat and light of combustion. It has been said that the elements formed the bricks while his new views on calcination and combustion formed the blueprint. The Traité itself formed a dualistic compositional edifice. Whenever an acidic earth and metal oxide (or earth) combined, they produced a salt, the oxygen they shared constituting a bond of union between them. As was appropriate for an elementary text, part III, a good third of the book, was devoted to chemical instrumentation and to the art of practical chemistry.
Lavoisier’s table of elements did not include the alkalis, soda and potash, even though these had not been decomposed. Why were they excluded from his pragmatic definition of simple substances? Two reasons have been suggested. In the first place, he was prepared to violate his criterion because of the chemical analogy between these two alkalis and ‘ammonia’, which Berthollet had decomposed into azote (nitrogen) and hydrogen in 1785. Lavoisier was so confident that soda and potash would be similarly decomposed into nitrogen and other unknown principles, that he withheld them from the table of simple substances. On the other hand, although confident that muriatic acid was also compound, because the evidence was not so strong as for the alkalis, he included it in the list of elements. While we may admire Lavoisier’s prescience – Davy was to decompose soda and potash in 1808 – this was a disturbing violation of his own pragmatism. What guarantee did the chemist have that any of Lavoisier’s simple substances were really simple? As we shall see, Lavoisier’s operational approach caused a century of uncertainty and helped to revive the fortunes of the ancient idea of primary matter.
A second explanation is more subtle. Lavoisier’s simple substances were arranged into four groups (see table 3.2). Three of the groups contained the six non-metals and seventeen metals then known, both of which were readily oxidizable and acidifiable, together with the group of five simple ‘earths’. The remaining group was light, caloric, oxygen, azote (nitrogen) and hydrogen. At first glance these elements appear to have nothing in common, but the heading Lavoisier gave them, ‘simple substances belonging to all the kingdoms of nature, which may be considered the elements of bodies’, provides the clue. Lavoisier probably saw these five elements as ‘principles’ that conveyed fundamental generic properties. Light was evidently a fundamental principle of vegetable chemistry; caloric was a principle of heat and expansibility; oxygen was the principle of acidity; hydrogen was the principle of water that played a fundamental role in all three kingdoms of Nature; and nitrogen was a principle of alkalinity. If the 1789 list of elements is compared with a preliminary list he published in 1787, it is found that azote was moved from its original position among the non-metals. It is not unlikely that this change was connected with the decomposition of ammonia and Lavoisier’s decision that soda and potash were compounds of ‘alcaligne’, a nitrogenous principle of alkalinity.
If this interpretation is correct, it illustrates again the role of continuity in Lavoisier’s revolutionary chemistry. Although we cannot now know if this was the position Lavoisier held – a position that was in any case subject to refutation and modification within a few years – it is intriguing to notice that organic chemists (beginning with Liebig) came to see certain elements, namely hydrogen, oxygen, carbon and nitrogen, as the ‘universal’ or ‘typical’ elements of mineral, animal and vegetable chemistry. It was on the basis of this that Gerhardt and Hofmann were to build a ‘type theory’ or organic classification and from which Mendeleev was to learn to classify a greatly extended list of elements in 1869.
By the mid 1790s the anti-phlogistonian camp had triumphed and only a few prominent chemists, such as Joseph Priestley, continued as significant critics. Unfortunately, by then the French Revolution had put paid to the possibility that Lavoisier would apply his insights to fresh fields of chemistry.
THE AFTERMATH
Although opposition to Lavoisier’s chemistry remained strong in Germany for a decade or more, largely for patriotic reasons, and although Cavendish and Priestley never converted, the speed of its uptake is impressive. Much depended, of course, on key teachers. In Germany, Sigismund Hermstadt (1760–1832) translated the Traité in 1792, and in the same year Christoph Girtanner (1760–1800) published a survey of Lavoisier’s chemistry. At Edinburgh the French-born Joseph Black, who had always taught that phlogiston was a principle of levity, lectured on the new chemistry while not necessarily committing himself to it until 1790. His successor, Thomas Charles Hope (1766–1844), ensured that large audiences of medical students learned the new theory after 1787. Scottish opposition seems to have been largely confined to geology, where James Hutton found phlogiston more accommodating to his theory that it was solar light and his need for a plutonic ignitor in the absence of oxygen deep inside the earth; and animal physiology, where, despite Lavoisier’s view of animal heat as the natural exothermic product of burning food inside the body, Adair Crawford developed a complex mechanism involving air, heat, blood, phlogiston and the specific heat capacity of blood.
Despite Lavoisier’s continued research after 1789 – for example, he began some promising work on the analysis of organic substances – he found his official activities as an academician and fermier taking up more and more of his time as the Revolution, which broke out in that year, created more and more technical and administrative problems.
When Lavoisier was born, France was still a monarchy and power lay firmly in the hands of the Crown and aristocracy together with the Roman Catholic church. These two powerful and sometimes corrupt groups, or Estates, which were virtually exempt from taxation, were the landlords of the majority Third Estate of peasant farmers, merchants, teachers and bankers from whom France’s wealth was derived. Agricultural depression, a rise in population and a succession of expensive wars (including France’s intervention in the American War of Independence in 1778) led France towards bankruptcy in the 1780s. The only solution to this seemed to be to introduce a more equitable system of taxation, which, in turn, involved the reformation of political structure, including the reduction of King Louis XVI’s despotic powers.
On 14 July 1789 revolution broke out with the storming of the Bastille prison in Paris. In fear of their lives, Crown and aristocracy renounced their privileges, while a National Assembly composed of the Third Estate drew up the Declaration of the Rights of Man. National unity was short-lived, however, as the more radical Jacobins manoeuvred for political power and the downfall of the monarchy. War with Austria and Prussia was to prove the excuse for the King’s execution on 21 January 1793. In the period of terror and anarchy that followed, Lavoisier was to lose his life. For, despite his undoubted support for the initial phase of the Revolution and his hard work within the Academy in improving the quality of gunpowder or in devising the metric system in 1790, his services to France and his international reputation were, in the words of one historian, ‘as dust in the balance when weighed against his profession as a Fermier-Géneral’. On 24 November 1793 Lavoisier and his fellow shareholders (including his father-in-law) were arrested and charged, ludicrously, with having mixed water and other ‘harmful’ ingredients in tobacco, charging excessive rates of interest and withholding money owed to the Treasury.
Although later investigations by historians have revealed the worthlessness of these charges, they were more than sufficient in the aptly named ‘Age of Terror’ to ensure the death penalty. Even so, there is some evidence that Lavoisier, alone of the fermiers, might have escaped but for the evidence that he corresponded with France’s political enemies abroad. The fact that his correspondence was scientific did not, in the eyes of his enemies, rule out the possibility that Lavoisier was engaged in counter-revolutionary activities with overseas friends.
Lavoisier was guillotined on 8 May 1794. The mathematician Lagrange commented, ‘It required only a moment to sever his head, and probably one hundred years will not suffice to produce another like it.’ Following the centenary of the French Revolution in the 1890s, a public statue was erected to commemorate Lavoisier. Some years later it was discovered that the sculptor had copied the face of the philosopher, Condorcet, the Secretary of the Academy of Sciences during Lavoisier’s last years. Lack of money prevented alterations being made and, in any case, the French argued pragmatically that all men in wigs looked alike anyway. The statue was melted down during the Second World War and has never been replaced. Lavoisier’s real memorial is chemistry itself.
CONCLUSION
A rational reconstruction of what seem to have been the essential features of the ‘chemical revolution’ would draw attention to six necessary and sufficient conditions. First, it was necessary to accept that the element, air, did participate in chemical reactions. This was first firmly established by Hales in 1727 and accepted in France by Rouelle and Venel. Although Hales tried to explain the fixation of air by solids by appealing to the attractions and repulsions of Newtonian particle theory, there was no satisfactory explanation for its change of state. Secondly, it was necessary to abandon the belief that air was elementary. This was essentially the contribution of the British school of pneumatic chemists. Beginning in 1754 with Black, who showed that the ‘fixed air’ released from magnesia alba had different properties from ordinary air, and continuing through Rutherford, Cavendish and Priestley, it was found possible to prepare and study some twenty or more ‘factitious airs’ that were different from ordinary air in properties and density. Their preparation and study were made possible by the development of apparatus by Hales for washing air, the pneumatic trough, thus extending the traditional ‘alchemical’ apparatus of furnaces and still-heads that had hitherto largely sufficed in chemical investigations. Whether factitious airs were merely modifications of air depending upon the amounts of phlogiston they contained, or distinct chemical species in an aerial condition, or the expanded particles of solid and liquid substances, was decided by Lavoisier’s development of a model of the gaseous state.
The concept of a gas was a necessary third condition for the reconstruction of chemistry. By imaging the aerial state as due to the expansion of solids and liquids by heat, or caloric, Lavoisier brought chemistry closer to physics and made possible the later adoption of the kinetic theory of heat and the development of chemical thermodynamics. The balance pan had always been the principal tool of assayers and pharmacists, while the conservation of mass and matter had always been implicit in chemists’ rejection of alchemical transmutation and their commitment to chemistry as the art of analysis and synthesis. With the conceptualization of a whole new dimension of gaseous-state chemistry, however, it was necessary that chemical analysis and book-keeping should always account for the aerial state. Here was a fourth necessary condition that raised problems for phlogistonists when Guyton demonstrated conclusively in 1771 that metals increased in weight when they were calcined in air. Many historians, like Henry Guerlac, saw this as the ‘crucial’ condition for effecting a chemical revolution and the event that set Lavoisier on his path to glory.
Largely for pedagogic reasons, generations of historians, chemistry teachers and philosophers of science have interpreted the chemical revolution as hinging upon rival interpretations of combustion – phlogiston theory versus oxygen theory. More recently, those historians who have seen Lavoisier’s chemistry as literally an anti-phlogistic chemistry have had a wider agenda than combustion in mind. In particular, it now seems clear that the interpretations of acidity was a major issue for Lavoisier and the phlogistonists. Indeed, it could be argued that, once Lavoisier had the concept of a gas, it was the issue of acidity, not combustion, that led him to oxygen – as its very name implies. The transformation of ideas of acidity, therefore, formed a fifth factor in the production of a new chemistry.
Finally, and not least, the sixth necessary condition was a new theory of chemical composition and organization of matter in which acids and bases were composed from oxygen and elements operationally defined as the substances that chemists had not succeeded in analysing into simpler bodies. Oxygen formed the glue or bond of dualistic union between acid and base to form salts, which then compounded in unknown ways to form minerals. To make this more articulate and to avoid confusion with the unnecessary thought patterns of phlogiston chemistry, a new language was required – one that reflected composition and instantly told a reader what a substance was compounded from. After 1787 chemists, in effect, spoke French, and this underlined the new chemistry as a French achievement.
Although he pretended at the beginning of the Traité that it had been his intent to reform the language of chemistry that had forced the reform of chemistry itself, it was clearly because he had done the latter that a new language of composition was needed. As historians have stressed, the new nomenclature was Lavoisier’s theoretical system. He justified its adoption in terms of Condillac’s empirical philosophy that a well constructed language based upon precise observation and rationally constructed in the algebraic way of equal balances of known and unknown would serve as a tool of analysis and synthesis.
Observation itself involved chemical apparatus – not merely the balance, but an array of eudiometers, gasometers, combustion globes and ice calorimeters, which would enable precise quantitative data to be assembled. In this way chemical science would approach the model of the experimental physicists that Lavoisier clearly admired and with whose advocates he frequently collaborated.
This last point has led some historians to question whether Lavoisier was a chemist at all and whether the chemical revolution was instead the result of a brief and useful invasion of chemistry by French physicists. Others, while admitting the influence of experimental physics on Lavoisier’s approach, continue to stress Lavoisier’s participation in a long French tradition of investigative analysis of acids and salts to which he added a gaseous dimension. Even Lavoisier’s choice of apparatus, though imbued with a care and precision lacking in his predecessors’ work, was hallmarked by the investigative procedures of a long line of analytical and pharmaceutical chemistry. All historians agree, however, that until about 1772, when events triggered a definite programme of pneumatic and acid research in his mind, Lavoisier’s research was pretty random and dull, as if he were casting around for a subject (‘une belle carrière d’expériences à faire’) that would make him famous. Seizing the opportunity, the right moment, is often the mark of greatness in science. Priestley and Scheele believed that science progressed through the immediate communication of raw discoveries and ‘ingenious simplicity’. Lavoisier’s way, to Priestley’s annoyance, was to work within a system and to theorize in a new language that legislated phlogiston out of existence.




