
Полная версия
The Fontana History of Chemistry
Rouelle’s pupil, G. F. Venel, was one of the few French chemists to pursue Hales’ work before the 1760s. He argued that natural mineral waters were chemical combinations of water and air, and that seltzer water could be reproduced by dissolving soda (sodium carbonate) and hydrochloric acid in water. He also advocated that the reactions of air had to be subsumed ‘under the laws of affinity’. In this way, air came to occupy one of the columns of the many dozens of different affinity tables that were published during the middle of the eighteenth century.
Lavoisier’s earliest knowledge of contemporary ideas concerning the elements, acidity, air and combustion was probably derived from Rouelle’s lectures, which he attended in 1762, as well as from Macquer’s Élémens de chymie théorique (1749) and Venel’s article on ‘chemistry’ in the third volume of the great French Encyclopédie (1753). Between them, Rouelle, Macquer and Venel turned their backs on Boyle’s seventeenth-century physical programme of attempting to reduce chemistry to ‘local motion, rest, bigness, shape, order, situation and contexture of material substances’. Instead, inspired by Newton, they intended to fuse the corpuscular tradition with the more pragmatic chemical explanations of Stahl. They also introduced Lavoisier to the quantitative analysis of minerals.
During the 1750s and 1760s the French government became aware that industry was ‘pushed much further in England than it is in France’. Wondering whether Britain’s increasing wealth and prosperity from trade and manufacture came because ‘the English are not hindered by regulations and inspections’, the French commissioned a series of reports on their country’s industries and natural resources. This interest had several effects: there was a sudden wave of translations of, chiefly, German and Scandinavian technical works on mining, metallurgy and mineral analysis; with these works, part and parcel, came an awareness of the phlogistic theory of chemical composition; moreover, chemists who had trained in pharmacy and medicine, like Macquer, began to find their services in demand for the solution of industrial problems. Guettard had long cherished an ambition to map the whole of France’s mineral possessions and geological formations, and the government readily gave approval in 1763. Needing an assistant who could identify minerals, Guettard persuaded Lavoisier to join him on his geological survey, which lasted until 1766.
In their travels through the French countryside, Lavoisier paid particular attention to water supplies and to their chemical contents. One mineral that particularly interested him was gypsum, popularly known as ‘plaster of Paris’ because it was used for plastering the walls of Parisian houses. Why, Lavoisier wondered, did the gypsum have to be heated before it could be applied as a plaster? Since water could be driven from the plaster by further heating, it seemed that the water could be ‘fixed’ into the composition of this and other minerals – a phenomenon that Rouelle had already termed ‘water of crystallization’. He then showed that it was the loss of some of the fixed water that explained the transformation of gypsum into plaster by heating. Lavoisier was to find the idea of ‘fixation’ significant.
Although Guettard’s geological map of France was never published and Lavoisier’s geological work remained largely unknown to his contemporaries, the work on gypsum was presented to the Academy of Sciences in February 1765, when Lavoisier was twenty-two. With a clear, ambitious eye on being elected to the Academy, the year before he had entered the Academy’s competition for an economical way of lighting Parisian streets. (This was some forty years before coal gas began to be used for this purpose.) Although his involved, meticulous study of the illuminating powers of candles and oil and pieces of lighting apparatus did not win him first prize when the adjudication was made in 1766, his report was judged the best theoretical treatment. King Louis XV ordered that the young man should be given a special medal.
Thus by 1766, this ambitious man had succeeded in bringing his name before the small world of Parisian intellectuals. In the same year, two years before he reached his legal majority of 25, Lavoisier’s father made a large inheritance over to him. To further his complete financial independence, in 1768 Lavoisier purchased a share in the Ferme Générale, a private finance company that the government employed to collect taxes on tobacco, salt and imported goods in exchange for paying the State a fixed sum of money each year. Members received a salary plus expenses, together with a ten per cent interest on the sum they had invested in the company. Such a tax system was clearly open to abuse; consequently, the fermiers were universally disliked and were to reap the dire consequences of their membership of the company during the French Revolution. All the evidence suggests that Lavoisier’s motives in joining the company were purely financial and that, as political events moved later, he strove actively to rid the system of corruption and fraud. Unfortunately, Lavoisier’s later suggestion that the fermiers should beat the smugglers by building a wall around Paris for customs surveillance was to lead to hostility towards him, as may be gathered from the punning aphorism ‘Le mur murent Paris fait Paris murmurant’ (The wall enclosing Paris made Paris mutter).
In 1771, at the age of twenty-eight, Lavoisier further cemented his membership of the Ferme Générale by marrying the fourteen-year-old daughter of a fellow member of the company, Marie-Anne Pierrette Paultze (1758–1836). Despite their difference of age and their childlessness, their marriage was an extremely happy one. Marie-Anne became her husband’s secretary and personal assistant. She learned English (which Lavoisier never learned to read) and translated papers by Priestley and Cavendish for him, as well as an Essay on Phlogiston by the Irish chemist, Richard Kirwan. The latter was then subjected to a critical anti-phlogistic commentary by Lavoisier and his friends, which actually led to Kirwan’s conversion. She also took lessons from the great artist, Louis David, in order to be able to engrave the extensive illustrations of chemical apparatus that appeared in Lavoisier’s Elements. David, in turn, portrayed the Lavoisiers together.
Madame Lavoisier was also hostess at weekly gatherings of Lavoisier’s scientific friends – a role she continued after his execution. It was through such continuing social activities in her widowhood that she met the American physicist, Benjamin Thompson (1753–1814), better known as Count Rumford, whose experiments on the heat produced during the boring of cannon had led him to question the validity of Lavoisier’s caloric theory of heat. After rejecting the suits of Charles Blagden and Pierre du Pont (whose son, Irénée, was to found the huge American chemical company), widow Lavoisier married Rumford in 1805; but they soon proved incompatible and quickly separated. Madame Lavoisier is a good example of how, before the time when they enjoyed opportunities to engage in higher education and in independent scientific research, women played a discrete, but essential, role in the development of science. At a time when the well-off could afford domestic servants, wives and sisters had abundant leisure to help their scientifically inclined fathers, husbands and brothers in their researches.
As a rich and talented man, Lavoisier was an obvious candidate for election to the prestigious Academy of Sciences. Unlike the Royal Society, whose Fellows have always been non-salaried, the French Academy of Sciences was composed of eighteen working ‘academicians’ or pensionnaires. As civil servants, they were paid by the French government (until 1793, by the Crown) to advise the State and to report on any official questions put to them as a body. There were also a dozen honorary members drawn from the nobility and clergy, a dozen working, but unpaid, ‘associates’ (associée) and, to complete the pecking order, a further dozen unpaid assistants (élèves or adjoints). The Academy also made room for its retired pensioners and for foreign honorary associates.
Because of its tight restriction on the number of salaried members, and of members generally, election to the Academy was a prestigious event in the career of a French scientist. This accolade was in contrast to Britain’s Royal Society, which allowed relatively easy access to its fellowship by those with wealth or social status as well as those with scientific talent; consequently, its fellowship lacked prestige. Indeed, until its election procedures were reformed in 1847, fellowship of the Royal Society was not necessarily the mark of scientific distinction that it is today.
The three working grades of the Académie, together with its aristocratic honorary membership, clearly reflected the rigid hierarchical structure of eighteenth-century French society. In practice, the pensioners were allocated between the six sciences of mathematics, astronomy, mechanics, chemistry, botany and anatomy (or medicine). Biology and physics were added under Lavoisier’s directorship of the Académie in 1785. Like the Nobel prizes today, such a distribution frequently prevented the election of a deserving candidate because the most appropriate scientific section was full. There was also a tendency to elect or to promote on grounds of seniority rather than merit. Because membership was restricted, vacancies often led to intense lobbying for positions, factionalism, ill-feeling and sometimes (as with Lavoisier’s election as an associé in 1772) to the bending of rules. The repeated failure of the revolutionary, Jean-Paul Marat, who fancied himself an expert chemist, to gain admission in the 1780s, led him and others to oppose the Academy. Its close association with Royal patronage and its reflection of the ‘corrupt’ hierarchical structure of the ancien régime in any case made it inevitable that it would be suppressed by the revolutionary government in August 1793.
Although, as was to be expected for one so brash and young, Lavoisier failed on his first attempt to join the Academy in 1766, by a modest bending of the rules to create an extra vacancy for him, he was successfully admitted to the lowest rank of assistant chemist in 1768. His chief sponsor described him as ‘a young man of excellent repute, high intellect and clear mind whose considerable fortune permits him to devote himself wholly to science’. Any fears that his membership of the tax company would interfere with his role as academician were probably repressed by the thought that he would be able to entertain on a lavish scale!
Much of Lavoisier’s fortune was probably spent on the best scientific apparatus that money could buy. Some of his apparatus was unique and so complex that his followers were forced to simplify his experimental procedures and demonstrations in order to verify their validity. It should not be thought from this that Lavoisier threw money away on instruments unnecessarily. For example, when measuring the quantity of oxygen liberated from lead calx in 1774, he found that traditional glass retorts were unusable because the lead attacked the glass; clay retorts gave similarly erroneous readings because of their porosity; hence for precise volumetric measurements Lavoisier was forced to design and have made an airtight iron retort. Expense was justified, then, because of the new standard of precision that Lavoisier demanded in chemistry. In the Traité he recognized that economies and simplifications would be possible, ‘but this ought by no means to be attempted at the expense of application, or much less of accuracy’.
Lavoisier was to be a loyal servant of the Academy, by helping to prepare its official reports on a whole range of subjects including – to select from one biographer’s pagelong list – the water supply of Paris, prisons, hypnotism, food adulteration, the Montgolfier hydrogen balloon, bleaching, ceramics, the manufacture of gunpowder, the storage of fresh water on ships, dyeing, inks, the rusting of iron, the manufacture of glass and the respiration of insects. It has been pointed out that, without an ethic of service, such as was entailed in a centralized Royalist state, a privileged citizen such as Lavoisier would have had no incentive to involve himself in such a ‘dirty’ subject as chemistry.
THE CHEMISTRY OF AIR
The problem of the Parisian water supply came to Lavoisier’s attention during the year of his election to the Academy when the purity of water brought to Paris by an open canal was questioned. The test for the potability of water involved evaporating it to dryness in order to determine its solid content. But the use of this technique reminded academicians, including Lavoisier, of the long tradition in the history of chemistry that water could be transmuted into earth. Obviously, if this were the case, the determination of the solid content ‘dissolved’ in water would reveal nothing about its purity.
As we have seen, the transmutation of water into earth had been a basic principle of Aristotle’s theory of the four elements, and a crucial, experimental, factor in van Helmont’s decision that water was the unique element and basis of all things. Although by the 1760s most chemists could no longer credit that such an apparently simple pure substance as water could be transmuted into an incredibly large number of complicated solid materials, it was seriously argued by a German chemist, Johann Eller, in 1746 that water could be changed into both earth and air by the action of fire or phlogiston. For Eller this was evidence that there were only two elements, fire and water. The active element of fire acted on passive water to produce all other substances.
It seems clear from the design of Lavoisier’s experiment on the distillaton of water, which he began in October 1768, that he suspected that the earth described in Eller’s experiment (which he probably read about in Venel’s article on ‘water’ in the fifth volume of the Encyclopédie in 1755) was really derived from the glass of the apparatus by a leaching effect. By weighing the apparatus before and afterwards, and also weighing the water before and after heating continuously for three months, Lavoisier showed that the weight of ‘earth’ formed was more or less equal to the weight loss of the apparatus. Intriguingly, Lavoisier did not clinch his quantitative argument by analysing the materials in the sediment and showing that they were identical to those in glass. Moreover, since the correlation of weights was not exact, some room for doubt remained until two decades later when Lavoisier showed that water was composed of hydrogen and oxygen.
Enough had been done, however, to convince Lavoisier that Eller’s contention that water could be transmuted into earth was nonsense. This was reported to the Academy in 1770. He also surmised, under the influence of Venel’s views on the chemical dissolution of air in liquids and solids, that there was a more plausible explanation of water’s apparent change into vapour or air when heated – namely, that heat, when combined with water and other fluids, might expand their parts into an aerial condition. Conversely, when air was stripped of its heat it lost its voluminous free aerial state and collapsed into, or was ‘fixed’ into, a solid or liquid condition, just as Stephen Hales had found in the 1720s when analysing the air content of minerals and vegetables.
Lavoisier recorded these ideas in an unpublished essay on the nature of air in 1772. Here was the basis for a theory of gases – though at this juncture Lavoisier knew nothing at all of the work of Priestley and others on pneumatic chemistry. He was also, not surprisingly, still interpreting his model of the gaseous state in terms of phlogiston. When air was fixed1:
… there had to be a simultaneous release of phlogiston or the matter of fire; likewise when we want to release fixed air, we can succeed only by providing the quantity of fire matter, of phlogiston, necessary for the existence of the gaseous state [l’état de fluide en vapeurs].
Lavoisier was now clear that there were three distinct states of matter2:
All bodies in nature present themselves to us in three different states. Some are solid like stones, earth, salts, and metals. Others are fluid like water, mercury, spirits of wine; and others finally are in a third state which I shall call the state of expansion or of vapours, such as water when one heats it above the boiling point. The same body can pass successively through each of these states, and in order to make this phenomenon occur it is necessary only to combine it with a greater or lesser quantity of the matter of fire.
Moreover, it followed from the fact that metals disengaged ‘air’ when they were calcined, that metals contained fixed air:
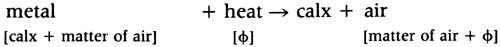
Apparatus for the preparation, collection and study of gases was a necessary factor in the chemical revolution. It was not until 1727 that Stephen Hales hit upon a way to isolate the ‘air’ produced from a heated solid. In order to estimate as accurately as possible the amount of ‘air’ produced and to remove any impurities from it, Hales ‘washed’ his airs by passing them through water before collecting them in a suspended vessel by the downward displacement of water.
Hales, like John Mayow in the seventeenth century, still thought in terms of a unique air element, but Joseph Black’s demonstration that ‘fixed air’ (carbon dioxide) was different from ordinary air encouraged Henry Cavendish, Joseph Priestley and others to develop Hales’ apparatus to study different varieties of air – or gases, as Lavoisier was to call them. An incentive here was the invention of soda water by Priestley, which encouraged interest in the potentially health-giving properties of artificial mineral waters generally. In 1765, while investigating spa waters, the English doctor, William Brownrigg, invented a simple shelf with a central hole to support a receiving flask or gas holder. This creation of the ‘pneumatic trough’ enabled gas samples to be transferred from one container to another and for gases to join solids and liquids on the chemical balance sheet.
Joseph Priestley (1733–1804) is surely one of the most engaging figures in the history of science. The son of a Yorkshire Congregational weaver and cloth-dresser, Priestley was trained for the Nonconformist ministry at a Dissenting academy in Daventry. Like most Nonconformist academies of the period, this taught a wider curriculum than the universities that included the sciences. After serving a string of ministries, where his theological views became increasingly Unitarian, and a teaching post at the famous Warrington Academy, in 1773 Priestley became the librarian and household tutor of William Petty, the second Earl of Shelburne who, while Secretary of State in Chatham’s cabinet, had opposed George III’s aggressive policy towards American colonists. Already the author of innumerable educational works, in 1767 Priestley had published a History and Present State of Electricity, which launched him upon a part-time career in science. While minister of a Presbyterian congregation at Leeds, and living next door to a brewery, Priestley had begun investigating the preparation and properties of airs. Under Shelburne’s patronage, Priestley had the necessary leisure to prepare some five volumes containing detailed accounts of these experiments on airs, as well as a number of theological works. There was a connection here in that Priestley was attempting to explore the relationship between matter and spirit.
In 1780, retaining a life annuity from Shelburne, Priestley returned to the ministry at Birmingham’s New Meeting. Here he found convivial philosophical and scientific company in the Lunar Society composed of rising industrialists and intellectuals such as Mathew Boulton, James Watt, Josiah Wedgwood and Erasmus Darwin. Although its members were united in their support for the American War of Independence and for the initial stages of the French Revolution, it was Priestley the preacher-orator who was publicly identified with radical criticism of English politics and the discrimination against Dissenters. In 1791 a ‘Church and King’ mob destroyed Priestley’s home and chapel, forcing him to flee to London. Although he was eventually compensated for the loss of his property, in 1794 he decided to emigrate and to join two of his sons in America.
Here he was warmly welcomed in Philadelphia, where he was offered the Chair of Chemistry at the University of Pennsylvania. Instead, Priestley moved to Northumberland, in rural Pennsylvania, where he hoped to found an academy for the sons and daughters of political refugees who would join him there. It did not work out and Priestley spent his declining years cut off by distance from European, and even American, intelligence, and fighting a rearguard action against Lavoisier’s chemistry in his fascinating Considerations on the Doctrine of Phlogiston (1796). Although outmanoeuvred by Lavoisier, Priestley lived on in two ways. His young executor, Thomas Cooper (1759–1839), a fellow refugee from English politics, acquired sufficient up-to-date knowledge of chemistry from studying in Priestley’s library and laboratory to become one of America’s leading chemical educators. A century after Priestley’s discovery of oxygen, in August 1874, a national meeting of chemists, gathered at his home in Northumberland (now a Priestley Museum), decided to create the American Chemical Society.
It was Cavendish who began the collection of water-soluble gases over mercury, but Priestley who brought their study and manipulation to perfection. Curiously, believing that chemistry, like physics, required expensive and complicated instruments, Lavoisier only rarely used the pneumatic trough; instead, he developed an expensive and sophisticated gasometer. A good third of Lavoisier’s Elements of Chemistry was devoted to chemical apparatus. Until the appearance of Michael Faraday’s Chemical Manipulation in 1827, Lavoisier’s descriptions remained the bible of instrumentation and chemical manipulative techniques.
In the spring of 1772, Lavoisier read an essay on phlogiston by a Dijon lawyer and part-time chemist, Louis-Bernard Guyton de Morveau (hereafter Guyton) (1737–1816). In a brilliantly designed experimental investigation, Guyton showed that all his tested metals increased in weight when they were roasted in air; and since he still believed that their combustibility was caused by a loss of phlogiston, he saved the phenomena by supposing that phlogiston was so light a substance that it ‘buoyed’ up the bodies that contained it. Its loss during decomposition therefore caused an increase of weight. Most academicians, including Lavoisier, thought Guyton’s explanation absurd. Following his previous reflections on the role of air, Lavoisier speculated immediately that a more likely explanation was that, somehow, air was being ‘fixed’ during the combustion and that this air was the cause of the increase in weight. It followed that ‘fixed air’ should be released when calces were decomposed – just as Hales’ earlier experiments in Vegetable Staticks had suggested.
One final Encyclopédie article seems to have influenced Lavoisier decisively at this juncture. This was an essay on ‘expansibility’ published in the sixth volume in 1756 by another pupil of Rouelle’s, the philosopher and civil servant, Jacques Turgot. Like Lavoisier, Turgot combined a career of public service with spare-time research in chemistry. But he never published his reflections (or if he did so, he did it anonymously), and we only know of his interesting thoughts from his private correspondence. Turgot arrived independently at the same solution as Lavoisier, namely that Guyton’s experiments could be explained as due to the fixing of air. He had actually learned of Guyton’s work before Lavoisier in August 1771. In a private letter to Condorcet, Turgot noted3:




