
Полная версия
The Teenage Brain: A neuroscientist’s survival guide to raising adolescents and young adults
“How could my [son/daughter] [fill in the blank]?”
Most of the parents who come up to me after a talk, or e-mail me or stop me at the grocery store, are exhausted or exasperated or both, and all of them could fill in the blank in that question with a whole host of perplexing actions, from “Why would my teenage daughter sneak out of the house in the middle of the night to be with her boyfriend after they just spent the whole weekend together?” to “How could my son raid the liquor cabinet of his friend’s parents—and then leave the empty bottles behind to boot?!”
A neighbor of mine with a sixteen-year-old was flummoxed when she caught her son smoking pot in his room when he was supposed to be studying. That was bad enough, she told me, but what astonished her even more was the fact that he had the window wide open (it was the middle of winter, mind you) in order to air out his room—and the wind was blowing the smoke back into the room, under the door and down the stairs, where it wafted toward my horrified friend in the kitchen!
“How could he be that stupid?” she asked me.
Parents quickly blame themselves for a teen’s poor behavior, even though they’re not exactly sure how or why they’re to blame. With biological parents, the guilt may come from passing on flawed DNA; and with biological and nonbiological parents or guardians, the guilt comes from questioning how they raised the child. In either case, you, the parent, are to blame, right? Yes, the two scenarios are different, but no, it’s not because of the genes or anything you did or didn’t do or because the teenager was somehow struck on the head and woke up as an alien species from the planet Adolescent.
Teenagers are different because of their brains and specifically because of two unusual aspects of their brains at this stage of their development. Their brains are both more powerful and more vulnerable than at virtually any other time in their lives. Even as they are learning things faster, their brains are eliminating gray matter and shedding neurons. How both of these facts can be true is because of something called neural plasticity.
Even as a teenager I used to wonder about the brain. Did it make a difference where a person grew up? How he or she grew up? Was the brain at all like the rest of the body—capable of changing depending on what went into it or what it was exposed to? I enjoyed turning these questions over in my head, and when I got to college they turned up again, only this time I began to have inklings of some of the answers.
During one summer while I was still in high school I volunteered at the Greenwich chapter of the Association for Retarded Citizens (ARC), now known simply as the Arc, which aids people with intellectual and developmental disabilities. Some of those who regularly attended the Greenwich ARC were born with Down syndrome, and though they had varying abilities, most were self-sufficient. They were able to swim and to participate in the theater program; some even learned to read and write. Because of Greenwich’s affluence, not only was the local ARC always well funded, but many of the kids came from very privileged backgrounds as well. To this day I remember being astonished when a limousine dropped off a tot for his day of activities with us. These children were really in an unusually enriched situation, and the effects of this gifted environment showed. Despite their handicaps and rather serious diagnoses, they were active and curious and engaged, and many were approaching milestones for reading and arithmetic close to those expected for normal kids their age. I knew that not only were they getting a great day at the ARC, but when they went home, they were often given physical therapy and tutoring there, too.
While at Smith, I had an opportunity to see what life was like for the mentally and developmentally disabled who did not have the same advantages as the children at the Greenwich ARC. I volunteered several hours a week at the Belchertown State School, a seventy-year-old state institution for the cognitively handicapped, located just a few miles from Smith. Belchertown’s residents ranged from children to the very elderly, many of whom had spent most of their lives at the institution. Before it closed in 1992, Belchertown housed as many as 1,500 people, ages one to eighty-eight, living in thirteen dormitories. The hospital was understaffed, even after a local newspaper exposed overcrowding and maltreatment in the 1960s. When I volunteered in 1975, I primarily spent time in the children’s dormitory. It was not a pleasant place. The rooms smelled of disinfectant, toys were few and far between, and many of the kids hadn’t been bathed in quite some time. Like the children at Greenwich’s ARC, some were more disabled than others, but even those who were more functioning seemed to lag far behind their peers at ARC. They sat in corners and rocked and had difficulty speaking, and their eyes appeared vacant.
This was a time at the height of the nature-versus-nurture debate, and my psychology and biology professors at Smith were keen on discussing how much a person’s makeup, from personality to intelligence to likes and dislikes, is dependent on genes (nature) and how much on the influence of environment (nurture). There was clearly little nurturing going on at Belchertown, while at ARC there were always activities, directed therapies, teaching, and, most of all, stimulation.
At some point I realized the children at Belchertown who had the same disabilities and the same hurdles to overcome were far worse off than the kids at ARC in Greenwich, and at least from my limited viewpoint, environment seemed to be the overwhelming determining factor. It was pure and simple: the brains of the ARC children were being stimulated and encouraged, and the brains of the Belchertown children were not.
Like fingerprints, no two brains are identical. Everything we do, think, say, and feel influences the development of our most precious organ, and those developments trigger ever more changes until the thread of action and reaction is too complex to unwind or undo. Our brains, in essence, are self-built. They not only serve the particular needs and functions of the particular individual, but also are shaped—landscaped if you will—by the individual’s particular experiences. In neuroscience, we refer to the human brain’s unique ability to mold itself as plasticity. Thinking, planning, learning, acting—all influence the brain’s physical structure and functional organization, according to the theory of neuroplasticity.
As far back as Socrates, some believed the brain could be “trained,” or changed, much as a gymnast trains his or her body to balance on a high beam. In 1942 the British physiologist and Nobel Prize winner Charles Sherrington wrote that the human brain was like “an enchanted loom, where millions of flashing shuttles weave a dissolving pattern, always a meaningful pattern, though never an abiding one.” In essence, the human brain, said Sherrington, was always in a state of flux.
Five years after Sherrington, Donald Hebb, an American neuropsychologist, was struck by a kind of accidental inspiration that led to the first quasi-experimental test of the theory of brain plasticity. When the forty-three-year-old researcher took rat pups home from his lab at Canada’s McGill University and gave them to his children as pets, he allowed the rodents to roam freely around the house. Hebb’s inspiration was to compare the brains of these free-roaming pet rats with those of rats kept in cages in his lab. After several weeks he put both groups of rats through a kind of intelligence test involving a maze. The pet rats, which had free access to explore the environment of Hebb’s home and unfettered interaction with one another as well as with Hebb and his family, performed significantly better on the maze test than the lab rats confined to small cages.
By the late 1990s researchers had confirmed a range of changes associated with experience and stimulation, including brain size, gray matter volume, neuron size, dendritic branching, and the number of synapses per neuron. The more stimulation and experience, they concluded, the larger the neurons, the bushier the dendrites, the higher the number of synapses, and the thicker the gray matter.
During my senior year at Smith College in 1977–78, I wrote my first professional journal article under the tutelage of Nico Spinelli, a professor in both the psychology department and the computer and information science department at the University of Massachusetts Amherst. He was doing pioneering experiments in the plasticity of the visual cortex. Previous research had looked at the brains of mammals raised in a deprived environment. Spinelli wanted to see if plasticity was still at work in a “normal” environment. So we took kittens raised with their mothers in a standard animal facility and gave them what’s called avoidance training. In these experiments, a “safe” and an “unsafe” stimulus were associated with two different visual stimuli: vertical lines and horizontal lines. As the kittens learned to associate the safe stimulus with either the horizontal or the vertical lines, the number of neurons in those parts of the visual cortex expanded. The results, which were published in the journal Science, confirmed “that early learning produces plastic changes in the structure of the developing brain,” or, to put it more simply, young brains are shaped by experience.
Of course, adult brains can be shaped by experience as well. Researchers in neural plasticity have found that even in the last decades of life, adult brains can be remodeled, just not as easily or as constantly as during childhood and adolescence. Whereas kids’ brains will respond and change in response to virtually any stimulation, so-called adult plasticity occurs only in specific behavioral contexts. For instance, cab drivers in London (a notoriously difficult city to navigate) have been found by scientists to have an enlarged hippocampus particularly in the area responsible for spatial memory. Violinists and cellists, who must use their hands fluidly and rapidly, have been shown to have an enhanced motor cortex. And in an unusual experiment conducted several years ago, Patricia McKinley of McGill University was able to show that learning the tango, which involves both complex movement and a fine sense of balance, improved the ability of senior citizens, ages sixty-eight to ninety-one, to switch between two different cognitive tasks. “Plasticity,” then, is just another way of saying “learning.”
In the first few years of childhood there is a critical period of plasticity in which learning comes quickly and easily. Evolution experts believe this is the brain’s way of helping us adapt early to the specific environment in which we are raised. The concept is the same as that of imprinting, whereby a baby duckling develops a keen and powerful preference to follow the mother duck over any other. When I was five years old, I saw this in action, although I obviously didn’t know it at the time. It was Easter, and my baby brother had just been born. Perhaps because of that, friends of my parents gave me my own “baby”—a baby chick, that is, much to my parents’ consternation. I loved that fuzzy little animal and was absolutely fascinated that it would follow me around the house, through the swinging door between the kitchen and the dining room, even out of the house and around the yard. Because I was with the chick almost from its birth, it had determined I was its mother. Years later I would read the children’s book Are You My Mother? by P. D. Eastman to my sons. Basically, the book is really all about imprinting. A young hatchling leaves its nest too early while its mother is out foraging for food, and goes on a journey, asking every animal and object it meets—a kitten, a hen, a dog, a cow, a car, even an enormous power shovel—the question of the title. Luckily the power shovel lifts the young bird up and deposits it back in its nest beside its real mother.
Five-year-old me, of course, was the only mother my baby chick had. Unfortunately, the end of the relationship was sudden and brutal. About a week after Easter, after I’d just gotten home from kindergarten, my baby chick was once again following me all over the house, but this time, as I skipped between the kitchen and the dining room, the little hatchling failed to make it through the swinging door and was squished. I cried for days.
Thirteen years later, as a freshman at Smith, I created my own chick-imprinting experiment for a class in advanced biology. In order to imprint them to sound, I exposed my baby chicks to a specific sound or tone every day over a week. At the end of this training period, the chicks were placed on a kind of runway and were then exposed to two sounds, one of them being the familiar tone I’d played for them for seven straight days. Every one of the chicks toddled toward the familiar tone: they had imprinted to sound. I remember this so well because my mother was visiting me at the time of the experiment and she helped me type the results!
But how does learning actually happen? Young brains and old brains work much the same way, by receiving information from the senses—hearing, seeing, tasting, touching, smelling. Sensory information is transmitted by synapses through a network of neurons and is stored, temporarily, in short-term memory. This short-term memory region is highly volatile and is constantly receiving input from the nearly continuous information our senses encounter every minute of our waking life. After information is processed in the short-term memory region, it is compared with existing memories, and if the information matches, it is discarded as redundant. (Brain space is too limited and too precious to allow duplicates to take up neural real estate.) If, on the other hand, the information is new, then it is farmed out to one of several locations in the brain that store long-term memories. Although nearly instantaneous, the transmission of sensory information is not perfect. In the same way that the otherwise seamless signal coming from your TV is occasionally interrupted, briefly distorting the picture, so, too, does degradation occur as information races up and down the axons of your brain’s neurons. This explains why our memories are never perfect, but have holes or discontinuities, which we occasionally fill in, albeit unconsciously, with false information.
The brain is programmed to pay special attention to the acquisition of novel information, which is what learning really is. The more activity or excitation between a specific set of neurons, the stronger the synapse. Thus, brain growth is a result of activity. In fact, the young brain has more excitatory synapses than inhibitory synapses.
The more a piece of information is repeated or relearned, the stronger the neurons become, and the connection becomes like a well-worn path through the woods. “Frequency” and “recency” are the key words here—the more frequently and the more recently we learn something and then recall it or use it again, the more entrenched the knowledge, whether it’s remembering the route between home and work or how to add a contact to your smartphone’s directory. In both cases, the mental machinery of learning is dependent on the synapse, that minuscule space where packets of information are passed from one neuron to another by electrical or chemical messengers. For these neural connections to be made, both sides of a synapse need to be “on,” that is, in a state of excitation. When an excitatory input exceeds a certain level, the receiving neuron fires and begins the molecular process, called long-term potentiation, by which synapses and neuronal connections are strengthened. The process of long-term potentiation, or LTP, is a complex cascade of events involving molecules, proteins, and enzymes that starts and ends at the synapse.
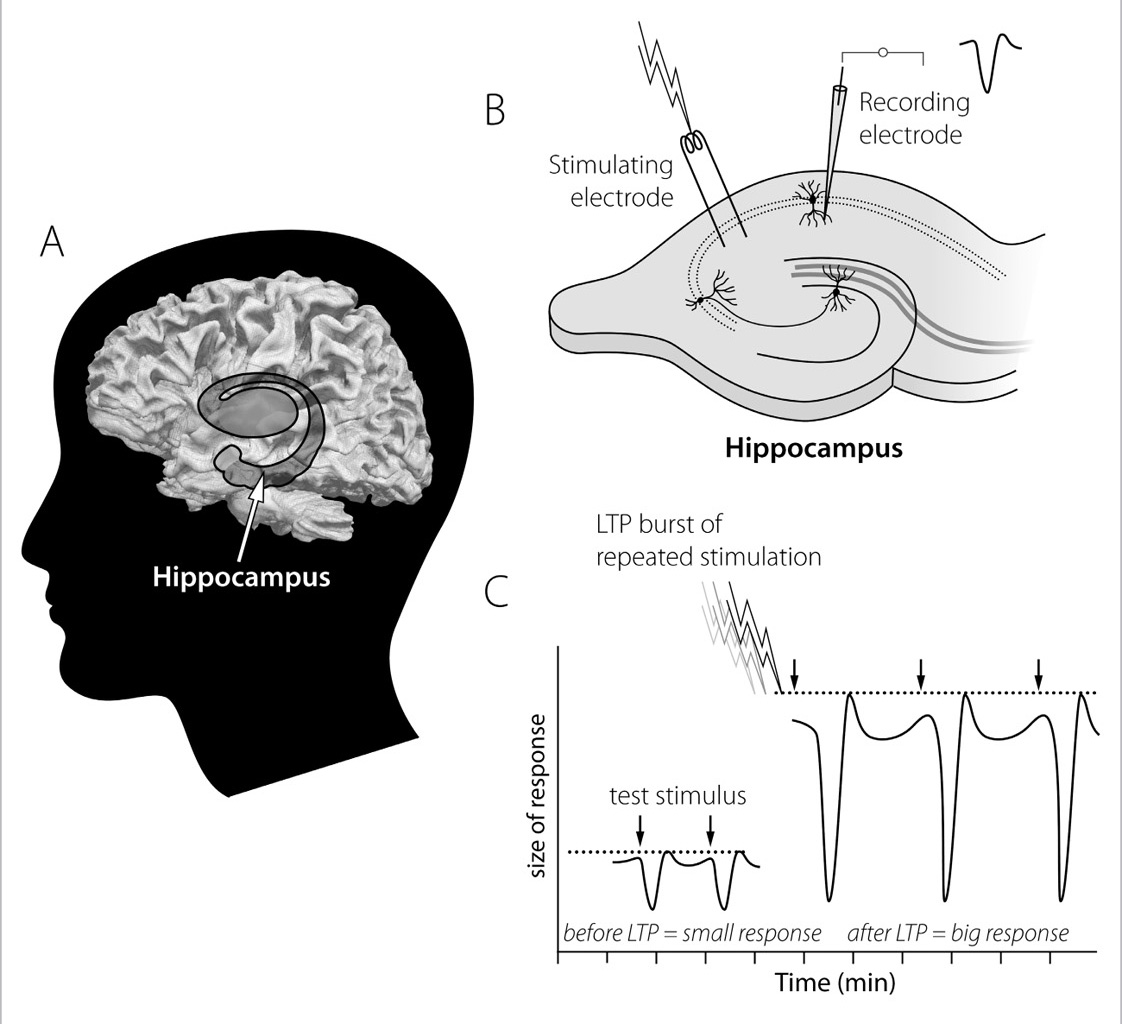
FIGURE 10. Long-Term Potentiation (LTP) Is a Widely Used Model of the “Practice Effect” of Learning and Memory: A. The hippocampus is located inside the temporal lobe. B. Brain cell activity recorded in hippocampal slices from rodents shows changes in cell signals after a burst of stimulation. C. LTP experiments commonly record repeated small responses to stimuli until a burst is given (akin to the “practice effect”), after which point responses from the neuron to the original stimulus become much larger, as if “memorized” or “practiced.”
The process of LTP begins with the main excitatory neurotransmitter, glutamate, being released at the axon terminal of one neuron across the synapse to the receptor on the dendrite of the receiving neuron. Glutamate is directly involved in building stronger synapses. How does it do this? Glutamate acts as a catalyst and sets off a chain reaction that eventually builds a bigger and stronger synapse, or connection in a brain pathway. When glutamate “unlocks” the receptor, it triggers calcium ions to zip around the synapse. The calcium, in turn, activates many molecules and enzymes and interacts with certain proteins to change their shape and behavior, which in turn can change the structure of synapse and neuron to make them more or less active. Calcium can alter existing proteins very rapidly, within seconds to hours, and it can also activate genes to make new proteins, a process that can take hours to days. The end result is a synapse that is bigger and stronger and that can cause a bigger response in the activated cell. In experiments, this increased response can be measured electrically as a bigger signal. Compared with the response before the “training” and the consequent building of a stronger synapse, the response in the cell after this strengthening, or potentiation, is much larger, and these measurements are the typical ones used in LTP experiments. In fact, if you are learning any of this at all, you are building new synapses as you read. Only minutes after you learn a new thing, your synapses start to grow bigger. In a few hours they are virtually cemented into a stronger form.
John Eccles, who would go on to win a Nobel Prize for his early work in the study of synapses, was perplexed by how much stimulation was needed to produce a synaptic change. “Perhaps the most unsatisfactory feature of the attempt to explain the phenomena of learning,” he wrote, “is that long periods of excess use or disuse are required in order to produce detectable synaptic change.” What Eccles failed to realize is that the repetitions he observed so frustratingly—those “long periods of excess use”—were the brain at work, learning and acquiring knowledge. After repeated stimulation, a brain cell will respond much more strongly to a stimulus than it initially did. Hence, the brain circuit “learns.” And the more ingrained the knowledge, the easier it is to recall and use. As when skiers race through a slalom course, the quickest route down becomes worn by use. Ruts develop. By the time the last competitors race through the gates, the route is so deeply entrenched in the snow that they can’t ski out of it, nor do they need or want to. The deeply imprinted line, in fact, guides them down without their having to search for it.
Конец ознакомительного фрагмента.
Текст предоставлен ООО «ЛитРес».
Прочитайте эту книгу целиком, купив полную легальную версию на ЛитРес.
Безопасно оплатить книгу можно банковской картой Visa, MasterCard, Maestro, со счета мобильного телефона, с платежного терминала, в салоне МТС или Связной, через PayPal, WebMoney, Яндекс.Деньги, QIWI Кошелек, бонусными картами или другим удобным Вам способом.


