
Полная версия
The use of accelerators and the phenomena of collisions of elementary particles with high-order energy to generate electrical energy. The «Electron» Project. Monograph
The world of elementary particles, micro-objects and quanta is amazing in its structure, way of existence and laws. Knowing the structure of matter, one inevitably has to accept the fact that the structure of any matter in the vicinity itself is a separate world, as already mentioned. Today, the theory of atomism is already widely known, which believed that everything in the world consists of the smallest particles – atoms. And if for the first time these ideas began since the time of Leucippus, Plato, Aristotle and many other scientists of antiquity, in whose time these thoughts mostly did not go beyond philosophical conclusions. However, as in the days of such great scientists as Abu Rayhan Biruni, Abu Ali ibn Sina, Al-Khorezmi, Ahmad Al-Khorezmi and other scientists of the East.
So there was even a time when atomism was even banned. And finally, when Sir Isaac Newton himself, along with other scientists, defended this grandiose idea, it began to be recognized and active research in this area began. But for a complete victory and proof of the reality of the existence of atoms, it was necessary to present some experimental evidence. Many scientists like John Dalton, Dmitry Ivanovich Mendeleev, Jean Perrin and many others tried to conduct this experiment, until finally Jean Perrin conducted his experiment with gummigut emulsion. By drawing an analogy of the change in the number of gummigut particles with the change in atmospheric pressure in height, Perrin was able to determine the weight of an atom for the first time.
And after the atom was fully recognized as an existing particle, work began to determine its structure. And now, after a series of studies and experimental confirmations by such brilliant experimental scientists and theorists as John Thompson, Ernest Rutherford, Niels Bohr and many others, the structure of the atom has been determined. And today it is proved not only with the help of indirect experiments, but also with the help of direct experimental evidence, a vivid example of which is the presence of a real photograph of an atom today, that the atom has a clear and clear structure.
But how can we come to this structure? It is worth dwelling on this issue in a little more detail. As you know, all objects are electrified, exchange charges, but where are they located? If all bodies have charges, including dielectrics (albeit small), therefore, charges are present in the structure of matter. Matter, as has already been proven, consists of molecules, and those of atoms, therefore, charges are inside atoms.
And the story of the discovery of the structure of the atom begins in 1897, when Joseph John Thompson discovered electrons while studying electric current in gases. That is, when a current was passed in a tube in which there were two electrodes – the cathode and the anode, the cathode emitted some rays, the so-called «cathode rays», the honor of accurately determining the type of these rays belongs to Mr. Thompson, who, by deflecting them in a magnetic field, as well as accelerating them in an electric field, established that this nothing else but some particles emitted by the cathode, with a negative electric charge, which is why they were called electrons.

Figure 2.1. Joseph John Thomson
And subsequent studies have led to the conclusion that electrons are part of an atom and when they fly out under the influence of an electric field, this leads to the transformation of an atom into an ion. But an ordinary atom is electrically neutral, therefore, in order to balance this charge, there must be a part with a positive charge in the atom. That is, an atom consists of charges that interact in some way. How does this interaction appear and is this interaction an explanation of the behavior of atoms in chemical reactions, in reactions with absorption and emission of light with certain wavelengths. After all, atoms may well be light sources, the same discharged gas emits light with certain spectra, at strict wavelengths, and how is this explained with the help of these interactions?
To explain this, in 1902, Mr. William Thompson, better known as Lord Kelvin, proposed his model of the structure of the atom, and already John Thompson studied it in more detail, so this model is known as the Thompson model. This model was popular until 1904 and is better known as the «raisin pudding model». According to this model, the atom consists entirely of positive matter, and electrons are inside it, moving freely. And with the help of this model, it was quite possible to describe some of the results.
Figure 2.2. William Thompson or Lord Kelvin

Figure 2.3. The Thompson model of the hydrogen atom
For example, you can describe a hydrogen atom. If we imagine a hydrogen atom in such a model, then the electron will «float» in a positive charge, but it will be pulled to the center of this positive «drop», due to the force of electrostatic equilibrium. If we assume that the electron departs from the center by a certain radius smaller than the radius of the atom itself, then it will be attracted by the mental sphere formed by this radius. But since it is charged uniformly, it can be concentrated in the center and simply written using the Coulomb formula (2.1).

And to determine the charge of an imaginary sphere formed inside a common large charge, you can use the ratio of this imaginary sphere to the entire sphere, and since the charge of the common sphere is already known and equal to the charge of the electron so that the atom is neutral, then the expression (2.2) is obtained, where the charge of the imaginary sphere is derived.
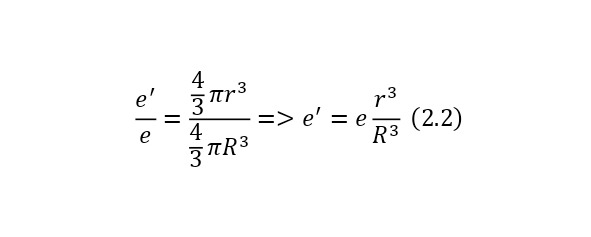
And if we already substitute this value for the Coulomb force, we get (2.3), a rather interesting expression that is directly proportional to the distance by which the electron moves away from the center.
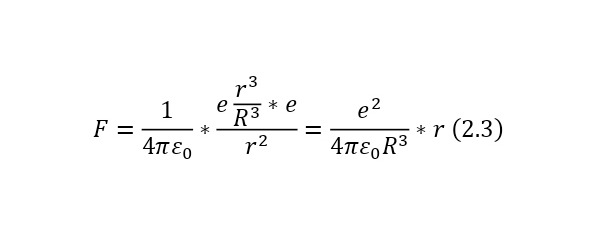
Also, for further convenience, we can introduce here the notion that the coefficient outside the radius of the imaginary sphere is the vibrational stiffness (2.4), and if we write with this stiffness not the Coulomb force formula itself, but its projection onto the radius of the imaginary sphere, then we get the expression (2.5), and negative, due to the fact that the vector the forces and the distance itself (the direction of the electron) are opposite.

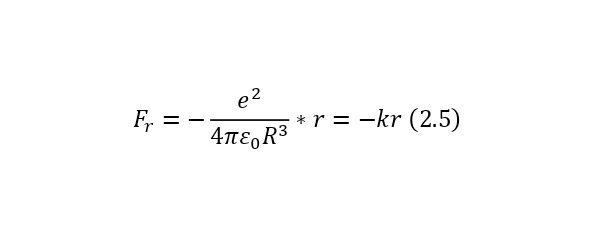
And now, if we assume that the electron oscillates in this way, then it resembles the construction of an oscillator or, more precisely, a mathematical pendulum with its rigidity and frequency determined by (2.6).
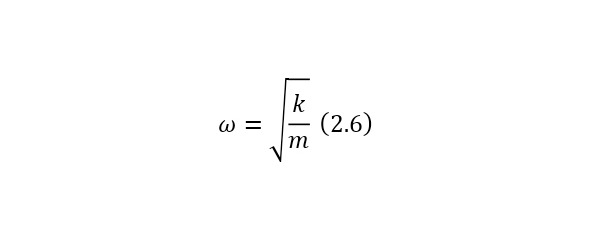
And if we substitute the necessary rigidity for (2.6), and take the mass of the electron as the mass, then the frequency will have the order of optical waves. That is, the atom glows in the visible region and even the glow effect can be explained using the Thompson model, but alas, another problem has arisen here. Even if we assume that the hydrogen atom glows, then according to this model it glows only with 1 frequency, when in reality it emits light with 4 frequencies. So it was proved that the Thompson model was not correct and it was necessary to create new models.
The next model is Ernest Rutherford's 1908-1910 model, which irradiated metal plates of thin gold foil with radioactive radiation, or more precisely with special alpha particles. At the same time, if you remove the plate on a circular screen (a phosphor that glowed), a point appeared, and when the plate was placed, this point scattered to form a spot, but in addition, some of these rays were reflected more than 90 degrees (right angle). And if we assume that the atom consists as the Thompsons were supposed to, then because of such a simply huge "smeared" positive charge on the size of the atom, the deviation should not have exceeded hundredths of a degree, and here there was a deviation of almost 180 degrees.
Then Rutherford suggested that in order to satisfy the results of the experiment, it should be assumed that the positive charge is strongly concentrated in a small area, and all the remaining space is practically empty, so the particles were only slightly scattered under the influence of an electric field or bumped into electrons that simply revolved around the atomic nucleus. This is how Rutherford first created a planetary model of an atom, according to which there is a single nucleus inside, and electrons already rotate around it in their orbits. However, there was still a lot to prove, for example, why did the electrons not fall to the atom, spending their energy on rotation, radiating energy at the same time?
But there was an answer to this question, thanks to Rutherford’s colleague Niels Bohr, who created the model of the hydrogen atom of Bohr, some postulates were accepted according to his model. Namely, the statements that an electron does not emit energy while in stationary orbits and can emit energy in the form of electromagnetic radiation (photons or light particles) only when moving from one orbit to another, and strictly with the energy equal to the energy difference in these two orbits. This has already led to the statement about the quantization of energy, that is, about operating with energy, particles, and their other parameters only in the form of portions. That is, there can be no smooth transition, either the electron is here, or it is not here, or it has released a certain amount of energy, or it has not. This idea was also supported by Max Planck when studying a «completely black body», a topic that would explain the glow when objects are heated.

Figure 2.4. Ernest Rutherford

Figure 2.5. Niels Bohr
Thus, when objects are heated, part of the energy from the collision of atoms flows to the nucleus, and after transferring it to an electron and its transition to another energy level, and then back, there is the release of a photon with a certain wavelength, so when bodies are heated, they emit light. And already when an external photon hits an atom, there is also an exit through the electron transition, but with a longer wavelength and, accordingly, a lower frequency, due to which such a phenomenon as absorption and reflection of light is observed. As for the passage of alpha particles during Rutherford's bombardment of gold foil, it was the nucleus with a high potential that caused such results, as well as the fact that almost 99.9% of the atom is empty and the same 99.9% of the atom's mass is concentrated in its nucleus. Thus, the Rutherford model was able to explain not only the results of the Rutherford experiment itself, but also many other phenomena, which confirms the validity of this model.
It is also appropriate to point out that the electrons are located not only in circular orbits, but also along their own separately defined paths, the shapes of which resemble "8" on different axes. This allows you to place a much larger number of electrons, for example, for such large atoms as uranium, with the ordinal number 92, neptunium-93, curium-96, californium-98 and many others. These paths are given from a separate theory of orbitals, which also proves the phenomenon of quantization in the world of elementary particles, from which it can be concluded that electrons do not move, however, like all micro-objects, they appear-disappear, appear-disappear, such is their nature of existence.
And all this forms the complete structure of the atom. This structure forms the so-called «quantum ladder», which is clearly manifested when determining the size of all particles. The atom itself has a diameter of about 10—8 cm, of course it differs from each atom, but the average size is equal to this indicator. In the center of the atom there is its own nucleus with a radius of about 10—12 cm. Electrons with a diameter less than 10—17 cm rotate around the nucleus, but this is a point particle for experimenters, since the exact size of the electron is difficult to consider at the moment and even when viewed with such an indicator as 10—17 cm, there will be no loss in accuracy. Unless you take into account experiments with increased accuracy aimed at studying higher resolutions.
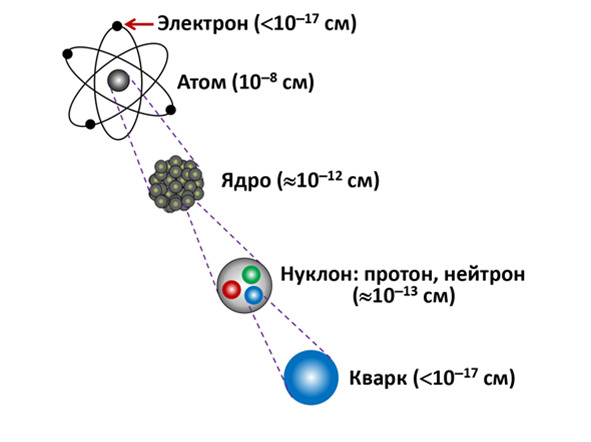
Figure 2.6. The quantum ladder
The nucleus itself is composite and consists of particles called nucleons, with further approximation it can be seen that there are 2 types of nucleons inside the nucleus: protons and neutrons. Each of them is approximately 10-13 cm in its own size . And with further approximation, smaller particles – quarks – can be observed. Quarks themselves are already point particles and have a size also smaller than 10-17, as well as electrons.
If we talk about further increase and passage even further into the depths of matter, then what will be there and how it looks is unknown today. But the fact is that it is quite difficult to do this even today.
And today the quantum world appears exactly in this form. Amazing operations are performed with these and many other particles, many other particles are formed. The study of the quantum world itself is very important, because today the study in this area has led to a number of discoveries, a vivid example of which is the creation of nuclear power plant technologies, the creation of particle accelerators, research in the field of thermonuclear reactions, widely known as "the creation of an artificial Sun" and many other studies have their origins in this area. And it was also in this area that the Electron research was born, to which this narrative is being conducted.
The discovery by Conrad X-Ray of special signals emitted by the cathode tube, which later received the name of the X-ray itself, caused a great furor. Many scientists began active research, but before the world could recover from this surprise, amazing materials that emitted these amazing rays were suddenly discovered. Henri Becquerel, who is one of the famous scientists who studied fluorescence, decided to prove the fact of the connection of this phenomenon with a radioactive source – uranium salt. It was then that Becquerel, in 1896, left the material on the photographic plate without illumination by chance and noticed that there were darkenings on the photographic plate, proving that the salt itself emits amazing rays. Many scientists have investigated this phenomenon until it was proved that these emissions are the result of radioactive decay of atomic nuclei.
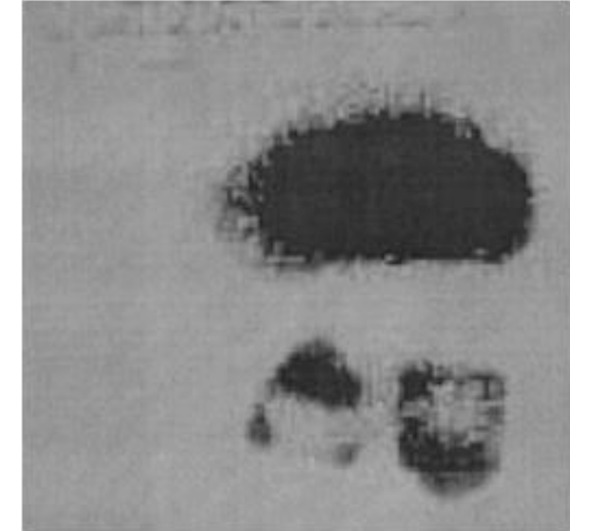
Figure 2.7 Photo taken by Becquerel
It is for this reason that 1896 is considered the year of the beginning of research in the field of the atomic nucleus. It was also known that if you direct focused radiation from a radioactive source (uranium salt) by placing it in a lead chamber with a single slit, and then place magnets on the path of this study, then this radiation will be divided into 3 types. At the same time, the radiation flux that was directed to the right has a negative charge, the flux that was turned to the left has a positive charge, which is easily proved from Lorentz's law. And the third radiation that has not been rejected has no charge.
Thus, the positive radiation was called alpha particles, and after measuring the masses of these particles based on the Lorentz force formula, when the magnetic field induction changes (the principle of operation of the mass spectrometer), it was possible to make sure that these are the nuclei of the helium atom. Negative particles, which were called beta particles, with the same analysis turned out to be just fast electrons, and rays that were not rejected were called gamma radiation.
After the initial analysis of the structure of radioactive radiation was carried out, it can be made sure that the radiation itself consists of 2 types of particles and 1 type of waves, namely gamma radiation, thanks to which it is already possible to give a general definition of radioactivity:
Radioactivity is the spontaneous emission of various particles and radiation by atomic nuclei.
Speaking in more detail about the dates of determination and research of radioactivity, it should be pointed out that by 1900 all types of radioactivity had already been investigated, although the atomic nucleus itself was discovered by Ernest Rutherford only in 1911. The first radiation, alpha radiation, which, as already determined, consists of helium nuclei, was discovered in 1898 by the same Ernest Rutherford and became known as alpha decay. Also beta decay or electron flight was discovered by the same Rutherford in the same 1898. But gamma radiation was determined and investigated only in 1900 by Paul Ulrich Willard.
These studies proved that the darkening of the plates observed by Becquerel was caused by radioactive radiation. Consequently, it is now possible to come to the concept of radioactive decay:
Radioactive decay is a spontaneous process characteristic of the phenomena of the microcosm at the quantum level. At the same time, the result of radioactive decay cannot be predicted accurately, only to determine the probability. Such a nature of phenomena is not an imperfection of devices, but is a representation of the processes of the quantum world themselves.
From this statement, we can conclude that there must be some generally accepted law explaining this phenomenon. The conclusion of the law of radioactive decay is as follows:
Let there be N (t) identical radioactive nuclei or unstable particles at a certain time t and the probability of the decay of a single nucleus (particle) per unit of time is equal to λ.
In this case, over a period of time dt, the number of radioactive nuclei (particles) will decrease by dN, which implies the following expression (2.7).
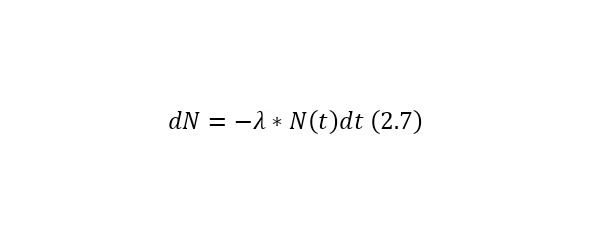
If we deduce a change in time from this ratio, we get (2.8).
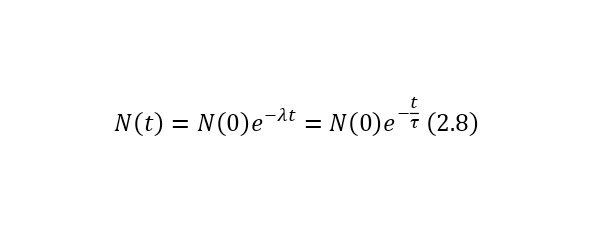
In (2.8), the concept of τ is defined in (2.9) and is the average lifetime of the nucleus (before decay), which is quite convenient to use, and N (0) in this case is the number of nuclei at the initial time.
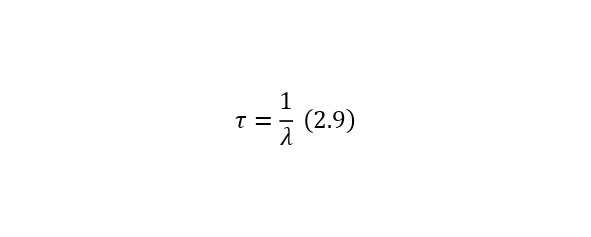
It is also possible to present another more simplified form (2.8) in (2.10).
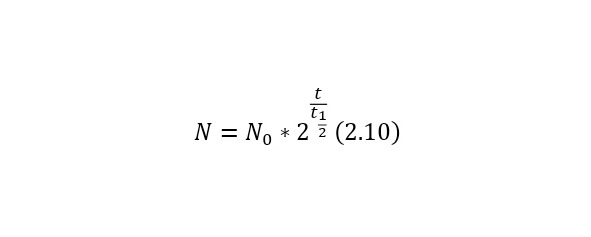
Where the half-index time is the half-life and is calculated by (2.11) and is equal to a separate value for each radioactive nucleus.

If it is necessary to determine the average number of decays (for low-speed decay), it is calculated by (2.12).
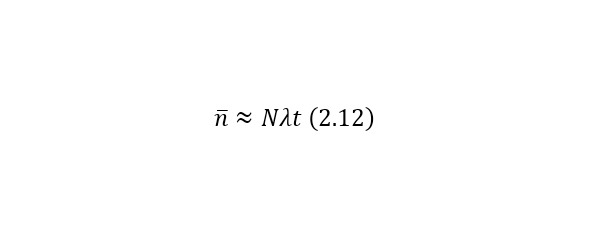
When this pattern is transformed, a radioactive decay curve is formed (Fig. 2.8).

Figure 2.8. Radioactive decay curve
From the graph, you can see that the pattern is exponential and at the same time decreases each time by half of the period, followed by a decrease.
As an experimental analysis of this phenomenon, the following can be shown. 100 measurements were carried out over the same period of time and the number of decays was measured. As a result, a graph was obtained on (Figure 2.9), where the average number of decays equal to 77.47 coincided with the value in (2.12), which is a clear proof of the validity of the general pattern.
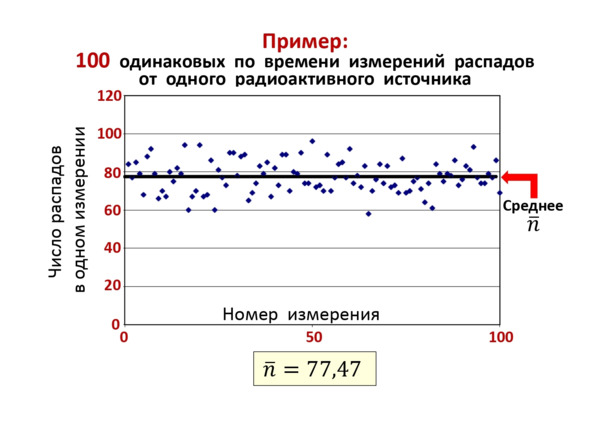
Figure 2.9. The result of the experiment
The general view of the distribution of these statistics is already presented according to a different law. That is, the probability Pn for the time t for testing the n number of decays is given by the Poisson distribution (2.13).
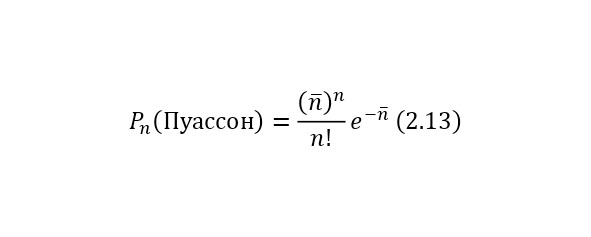
This conclusion is already inherent in probability theory, and if we rely on it, then also for the case when (n>> 1) Gaussian distributions (2.14) are already used.
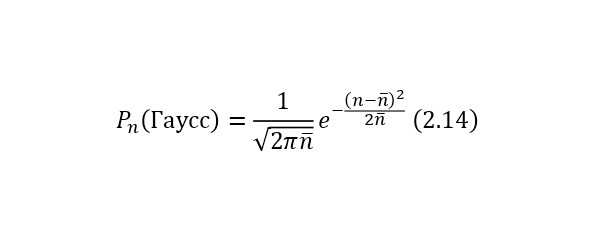
If we express these two patterns on graphs, we can get almost identical patterns with an increase in the average number of decays. For example, if the average number of decays is 2, then there is some difference in the results of the Poisson and Gauss distribution, but when this number, for example, reaches 7 and higher values, this difference becomes less significant, as shown in (Figure 2.10).

Figure 2.10. The graph of the probability of decay according to the Poisson and Gauss distributions for the average decay number equal to 2 and 7
After it has been decided with probability at zero speed, we can pay attention to cases when the effects of the theory of relativity come into play. In the microcosm, where the sizes of the studied objects are practically invisible, for example, for atoms with their sizes of 10-8 cm, for atomic nuclei with their 10-12-10-13 cm and for other particles with 10-13-10-17 cm, the speeds are often comparable, close or even equal to the speed of light. Thanks to this, all the features and effects of the theory of relativity are clearly manifested in the microcosm.
For this reason, it is important to consider in more detail the relations and basic equations from the theory of relativity.
One of the most important elements in the theory of relativity is the Lorentz factor (2.15), which is involved in almost all formulas of the theory of relativity, which can also be derived from the kinetic energy formula (2.16).
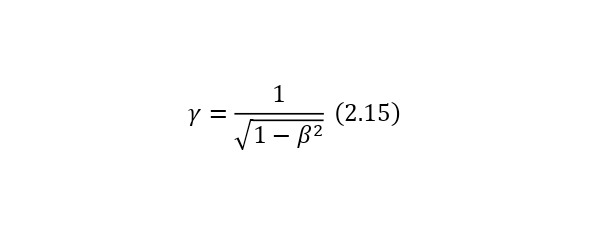
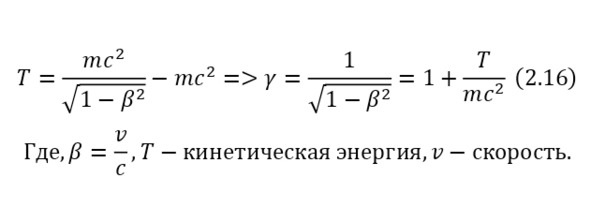
From these relations, it can be concluded that the total energy, which is the sum of the kinetic energy and the rest energy of the particle, is determined by (2.17).

The presence of this equality leads to the fact that the problem of the lack of a formula for calculating the energy of particles without masses (for example, a photon or a gluon) is solved. And already from (2.16) it is also possible to derive a more simplified entry for kinetic energy (2.18). In the case of applying (2.15) for the momentum formula (2.19), a simplified form is also obtained.
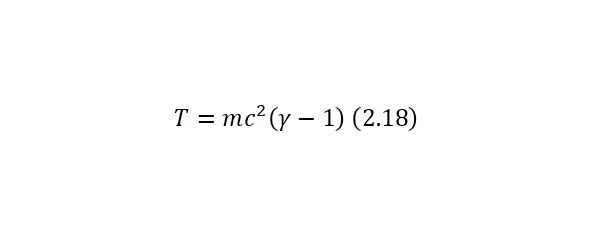

The velocity of the particle derived from the formulas of the total energy (2.17) looks like this (2.20).

