 полная версия
полная версияColour Measurement and Mixture
We can also brush a solution of quinine on the screen, and immediately the place where the ultra-violet rays fall is illuminated by a violet light. We do not see the ultra-violet rays themselves, but only the rays of increased wave-length, which are emitted by their effect on the sulphate of quinine. Common machine oil as used for engines also emits greenish rays when excited by the ultra-violet rays, and a very beautiful colour it is. Fluorescence then is one means of demonstrating the existence of the ultra-violet rays – or Ritter's rays as they were formerly called, after their discoverer – in a very simple manner. The method of rendering the effects of the infra-red rays visible to the eye is also interesting. All, or at all events most, of our readers have seen Balmain's luminous paint. A glass or card coated with this substance, which is essentially a sulphide of calcium, when exposed to the light of the sun, or of the electric arc, and then taken into comparative darkness, is seen to shine with a peculiar violet-coloured light. If when thus excited we place it in a bright spectrum for some little time, we shall find on shutting off the light that where the ultra-violet and blue fell on it, the violet light is intenser than the light of the main part of the screen; where the yellow fell there is neither increase or diminution in brightness; but that in the red it becomes darker, and also beyond the limit of the visible spectrum, indicating the existence of rays beyond, which through their greater length have not the power of affecting the eye. If the spectrum be shut off, however, very soon after it falls on the plate, it has been asserted that the red and infra-red rays have increased the brightness of that particular part of the plate on which they fell. At first these two observations seem to contradict one another; they do not in reality. We may expose a tablet of Balmain's paint to light, and place a heated iron in contact with the back of the plate; we shall then find that the iron produces a bright image of its surface on a less bright background. This bright image will gradually fade away, and the same space will eventually become dark compared with the rest of the plate. The reason of this is clear. When light excites the paint a certain amount of energy is poured into it, which it radiates out slowly as light. When the hot iron is placed in contact with it, the heat causes the light to radiate more rapidly, and consequently with greater intensity, at the part where its surface touches, and the energy of that particular portion becomes used up. When the energy of radiation of this part becomes less than that of the rest of the tablet, its light must of necessity be of less brightness than that of the background, with which the heated iron has had no contact. For this reason the image of the iron subsequently appears dark. We shall see presently, and as before stated, that the principal heating effect of the spectrum lies in the red and infra-red, and it is owing to the heating of the paint by these rays that the image might be at first slightly brighter than the background, and subsequently darker.
There is another way in which the existence of both the ultra-violet and infra-red rays can be demonstrated, and that is by means of photography. If we place an ordinary photographic plate in the spectrum and develop it, we shall find that besides being affected by the blue and violet rays, it is also affected by the rays beyond the violet, the energy of these rays being capable of causing a decomposition of the sensitive silver salt. If quartz prisms and lenses be used, and the electric light be the source of illumination, the ultra-violet spectrum will extend to an enormous extent. A more difficult, but perhaps even more interesting means of illustrating the existence of the infra-red rays, and first due to the writer, can be made by means of photography. It is possible to prepare a photographic plate with bromide of silver, which is so molecularly arranged that it becomes capable of being decomposed not only by the violet and blue rays, but also by the red rays, and by those rays which have wave-lengths of nearly three times that of the red rays. It would be inappropriate to enter into a description of the method of the preparation of these plates. Those who are curious as to it will find a description in the Bakerian lecture published in the Philosophical Transactions of the Royal Society for 1881. With plates so prepared it has been found possible to obtain impressions in the dark with the rays coming from a black object, heated to only a black heat.
That these dark rays possess greater energy or capacity for doing work of some kind than any other rays of the spectrum, can be shown by means of a linear thermopile (Fig. 4), if it be so arranged as to allow only a narrow vertical slice of light to reach its face.
Fig. 4. – The Thermopile.
The principle of the thermopile we need not describe in detail. Suffice it to say that the heating of the soldered junctions of two dissimilar metals (there are ten pairs of antimony and bismuth in the above instrument) produces a feeble current of electricity, which, however, is sufficient to cause a deflection to the suspended needle of a delicate galvanometer. To the needle is attached a mirror weighing a fraction of a grain, and the deflections are made visible by the reflection from it of a beam of light issuing from a fixed point along a scale. The greater the heating of the junctions of the thermopile, within limits which in these cases are never exceeded, the greater is the current produced, and consequently the greater is the deflection of the mirror-bearing needle, and of the beam of light along the scale. In order to get a comparative measure of the energies of the different rays, it is necessary that they should be completely absorbed. Now the junctions themselves of the pile being metal, and therefore more or less bright, will not absorb completely, but if they be coated with a fine layer of lamp-black, the rays falling on the pile will be absorbed by this substance, and their absorption will cause a rise in temperature in it, and the heat will be communicated to the thermopile.
If we make a bright spectrum, and one not too long, say three inches in length, and pass the linear thermopile through its length, we shall find that when the galvanometer is attached, the galvanometer needle will be differently deflected in its various parts. The deflection will be almost insensible in the violet, but sensible in the blue, rather more in the green, still more in the yellow, and it will further increase in the red. When, however, the slit of the thermopile is placed beyond the limit of the visible spectrum, the deflection enormously increases, and will increase till a position is reached as far below the red as the yellow is above it. After this maximum is reached, by moving the pile still further from the red, the galvanometer needle will travel towards its zero, and finally all deflection will cease. At this point we may suppose we have reached the limit of the spectrum, but if rock-salt prisms and lenses be used, the limit will be increased. What the real limit of the spectrum is, is at present unknown; Mr. Langley with his bolometer, and rock-salt prisms, an instrument more sensitive than the thermopile, must have nearly reached it.
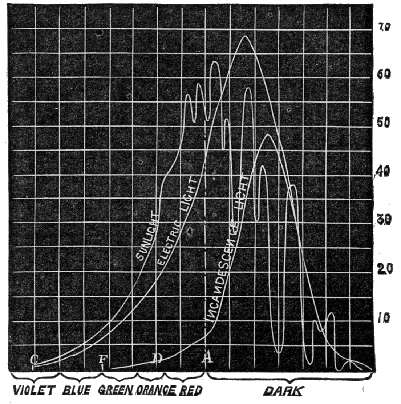
Fig. 5. – Heating effect of different Sources of Radiation.
The above figure is a graphic representation of the heating effect of the spectrum of the electric light, sunlight, and the incandescence electric light, on the lamp-black coating of the thermopile, as shown by the galvanometer. The vast difference between the heating effect of the visible rays of the first two sources compared with the last is clearly indicated.
Since every ray may be taken as totally absorbed, the heating of the lamp-black is a measure of the energy or the capacity of performing work of some description, which they possess. Waves of the sea do work when they beat against the shore, and they do work when they lift a vessel. If we notice a ship at anchor we shall find that behind the vessel and towards the shore the waves are lowered in height or amplitude; the energy which they have expended in raising the vessel of necessity causes this lowering. In the same way the waves of light, after falling on matter whose molecules or atoms are swinging in unison with them, are destroyed, and the energy is spent in either decomposing the matter into a simpler form at first – though the subsequent form may be more complex – or in raising its temperature. As lamp-black or carbon is in its simplest form, the only work done upon it by the energy of radiation is the raising of its temperature, and it is for this reason that this material is so excellent for covering the junctions of the pile. The eye evidently does not absorb all rays, since only a limited part of the spectrum is visible, and it would be useless to take a measure of the heating effect of lamp-black for the visible part of the spectrum as a measure of its luminosity, since the latter fades off in the red – the very place in which the heat curve rises rapidly.
CHAPTER IV
Description of Colour Patch Apparatus – Rotating Sectors – Method of making a Scale for the Spectrum.
Before proceeding further we must describe somewhat in detail two or three pieces of apparatus to be used in the experiments we shall make.
The first piece was devised by the writer a few years ago, and has got rid of several objections which existed in older pieces of apparatus. It is not only useful for lecture purposes, but also for careful laboratory work. The ordinary lecture apparatus for throwing a spectrum on the screen is of too crude a form to be effective for the purpose we have in view; the purity of the colours seen on the screen is more than doubtful, and this alone unfits it for our experiments. If we want to form a pure spectrum we must have a narrow slit, prisms with true, flat surfaces, and lenses of proper curvature. As a rule the ordinary lecture apparatus for forming the spectrum lacks all of these requisites.
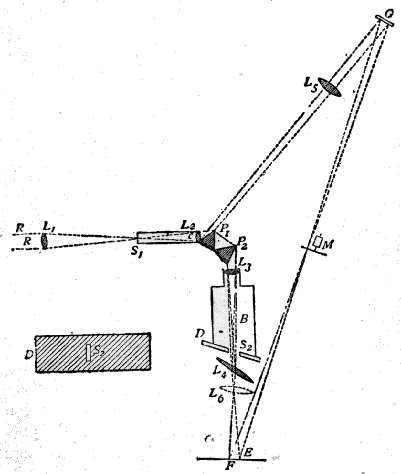
Fig. 6. – Colour Patch Apparatus.
The accompanying diagram (Fig. 6) will give an idea of the apparatus we shall employ. On the usual slit S₁ of a collimator C is thrown, by means of a condensing lens L₁, a beam of light, which emanates from the intensely white-hot carbon positive pole of the electric light. The focus is so adjusted that an image of the crater is formed on the slit. The collimating lens L₂ is filled by this beam, and the rays issue parallel to one another and fall on the prisms P₁ and P₂, which disperse them. The dispersed beam falls on a corrected photographic lens L₃, attached to a camera in the ordinary way. It is of slightly larger diameter than the height of the prisms, and a spectrum is formed on the focusing-screen D, which is slewed at a slight angle with the perpendicular to the axis of the lens L₃. This is necessary, because the focus of the least refrangible or red rays is longer than that of the more refrangible or blue rays. By slewing the focusing-screen as shown, a very good general focus for every ray may be obtained. When the focusing-screen is removed, the rays form a confused patch of parti-coloured light on a white screen F, placed some four feet off the camera. The rays, however, can be collected by a lens L₄, of about two feet focus, placed near the position of the focusing-screen, and slightly askew. This forms an image on the screen of the near surface of the last prism P₂; and if correctly adjusted, the rectangular patch of light should be pure and without any fringes of colour. The card D slides into the grooves which ordinarily take the dark slide. In it will be seen a slit S₂, the utility of which will be explained later on.
We shall usually require a second patch of white light, with which to compare the first patch. Now, although the light from the positive pole of the carbons is uniform in quality, it sometimes varies in quantity, as it is difficult to keep its image always in exactly the centre of the slit. If we can take one part of the light coming through the slit to form the spectrum, and another part to form the second patch of white light, then the brightness of the two will vary together. At first sight this might appear difficult to attain; but advantage is taken of the fact that from the first surface of the first prism P₁ a certain amount of light is reflected. Placing a lens L₅, and a mirror G, in the path of this reflected beam, another square patch of light can be thrown on the same screen as that on which the first is thrown, and this second patch may be made of the same size as the first patch, if the lens L₅ be of suitable focus, and it can be superposed over the first patch if required; or, as is useful in some cases, the two patches may be placed side by side, just touching each other.
We are thus able to secure two square white patches upon the screen F, one from the re-combination of the spectrum, and one from the reflected beam. If a rod be placed in the path of these two beams when they are superposed, each beam will throw a shadow of the rod upon the screen. The shadow cast by the integrated spectrum will be illuminated by the reflected beam, and the shadow cast by the latter will be illuminated by the former. In fact we have an ordinary Rumford photometer, and the two shadows may be caused to touch one another by moving the rod towards or from the screen. When the illumination of the two shadows by the white light is equal, the whole should appear as one unbroken gray patch. To prevent confusion to the eye a black mask is placed on the screen F with a square aperture cut out of it, on which the two shadows are caused to fall. If it be desired to diminish the brightness of either patch, it can be accomplished by the introduction of rotating sectors M, which can be opened and closed at pleasure during rotation, in the path of one or other of the beams.
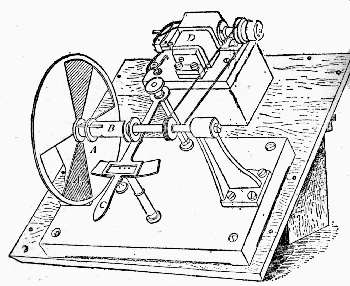
Fig. 7. – Rotating Sectors.
The annexed figure (Fig. 7) is a bird's-eye view of the instrument. A A are two sectors, one of which is capable of closing the open aperture by means of a lever arrangement C, which moves a sleeve in which is fixed a pin working in a screw groove, which allows the aperture in the sectors to be opened and closed at pleasure during their revolution; D is an electro-motor causing the sectors to rotate. To show its efficiency, if two strips of paper, one coated with lamp-black and the other white, are placed side by side on the screen, and if one shadow from the rod falls on the white strip, and the other shadow on the black strip of paper, and the rotating sectors are interposed in the path of the light illuminating the shadow cast on the white strip, the aperture of the sectors can be closed till the white paper appears absolutely blacker than the black paper. White thus becomes darker than lamp-black, owing to the want of illumination. This is an interesting experiment, and we shall see its bearings as we proceed, as it indicates that even lamp-black reflects a certain amount of white or other light.
Having thus explained the main part of the apparatus with which we shall work, we can go on and show how monochromatic light of any degree of purity can be produced on the screen. If the slit in the cardboard slide D be passed through the spectrum when it has been focused on the focusing-screen, only one small strip of practically monochromatic light will reach the screen, and instead of the white patch on the screen we shall have a succession of coloured patches, the colour varying according to the position the slit occupies in the spectrum. It should be noted that the purity of the colour depends on two things – the narrowness of the slit S₁ of the collimator, and of the slit S₂ in the card. If two slits be cut in the card D, we shall have two coloured patches overlapping one another, and if the reflected beam falls on the same space we shall have a mixture of coloured light with white light, and either the coloured light or the white light can be reduced in brightness by the introduction of the rotating sectors. If the rod be introduced in the path of the rays we shall have two shadows cast, one illuminated with coloured light, monochromatic or compound, and the other with white light, and these can be placed side by side, and surrounded by the black mask as before described.

Fig. 8. – Spectrum of Sodium Lithium and Carbon.
There is one other part of the apparatus which may be mentioned, and that is the indicator, which tells us what part of the spectrum is passing through the slit. Just outside the camera, and in a line with the focusing-screen, is a clip carrying a vertical needle. A small beam of light passes outside the prism P₁; this is caught by a mirror attached to the side of the apparatus, and is reflected so as to cast a shadow of the needle on to the back of the card D, on which a carefully divided scale of twentieths of an inch is drawn. To fix the position of the slit the poles of the electric light are brushed over with a solution of the carbonates of sodium and lithium in hydrochloric acid, and the image of the arc is thrown on the slit. This gets rid of the continuous spectrum, and only the bright lines due to the incandescent vapours appear on the focusing-screen (Fig. 8). Amongst other lines we have the red and blue lines due to the vapour of lithium; the orange, yellow (D), and green lines of sodium, together with the violet lines of calcium (these last due to the impurities of the carbons forming the poles). These lines are caused successively to fall on the centre of the slit by moving the card D, which for the nonce is covered with a piece of ground glass, and the position of the shadow of the needle-point on the scale is registered for each. A further check can be made by taking a photograph of these lines, or of the solar spectrum, and having fixed accurately on the scale any one of these lines already named, the position of the others on the scale may be ascertained by measurement from the photograph. Now the wave-lengths of these bright lines have been most accurately ascertained, in fact as accurately as the dark lines in the solar spectrum. Thus the scale on the card is a means of localizing the colour passing through the slit or slits. Should more than one slit be used in the spectrum the positions of each can be determined in exactly the same way. The most tedious part of the whole experimental arrangement with this apparatus is what may be called the scaling of the spectrum.
A fairly large spectrum may be formed upon the screen without altering any arrangement of the apparatus, when it has been adjusted to form colour patches. If a lens L₆ (see Fig. 6) of short focus be placed in front of L₄ (the big combining lens), an enlarged spectrum will be thrown upon the screen F, and if slits be placed in the spectrum the images of their apertures are formed by the respective coloured rays passing through them, so that the colours which are combined in the patch can be immediately seen.
CHAPTER V
Absorption of the Spectrum – Analysis of Colour – Vibrations of Rays – Absorption by Pigments – Phosphorescence – Interference.
We must now briefly consider what is the origin, or at all events the cause, of the colour which we see in objects. It is not proposed to enter into this by any means minutely, but only sufficiently to enable us to understand the subject which is to be brought before you. What for instance is the cause of the colour of this green solution of chlorophyll, which is an extract of cabbage leaves? If we place it in the front of the spectrum apparatus and throw the spectrum on the screen, we find that while there is a certain amount of blue transmitted, the green is strong, and there are red bands left, but a good deal of the spectrum is totally absorbed. Forming a colour patch of this absorption spectrum on the screen, we see that it is the same colour as the chlorophyll solution, and of this we can judge more accurately by using the reflected beam, and placing the rod in position to cast shadows. (The light of the reflected beam is that of the light entering the slit.) The colour then of the chlorophyll is due to the absence of certain colours from the spectrum of white light. When white light passes through it, the material absorbs, or filters out, some of the coloured rays, and allows others to pass more or less unaffected, and it is the re-combination of these last which makes up the colour of the chlorophyll. We have a green dye which to the eye is very similar in colour to chlorophyll, but putting a solution of it in front of the spectrum, we see that it cuts off different rays to the latter. It would be quite possible to mistake one green for the other, but directly we analyze the white light which has filtered through each by means of the spectrum, we at once see that they differ. Hence the spectrum enables the eye to discriminate by analysis what it would otherwise be unable to do. Any coloured solution or transparent body may be analyzed in the same way, and, as we shall see subsequently, the intensity of every ray after passing through it can be accurately compared with the original incident light. There are some cases, indeed the majority of cases, in which the colour transmitted through a small thickness of the material is different to that transmitted through a greater thickness. For instance, a weak solution of litmus in water is blue when a thin layer is examined, and red when it is a thicker or more concentrated layer. Bichromate of potash is more ruddy as the thickness increases. This can be readily understood by a reference to the law of absorption. Suppose we have a thin layer of a liquid which gives a purple colour when two simple colours, red and blue, pass through it, and that this thin layer cuts off one-quarter of the red and one-half of the blue incident on it, another layer of equal thickness will cut off another quarter of the three-quarters of red passing through the first layer, and half of the one-half left of the blue; we shall thus have nine-sixteenths of the red passing and only a quarter of the blue. With a third layer we shall have twenty-seven sixty-fourths of red and only one-eighth of blue left, showing that as the thickness of the liquid is increased the blue rapidly disappears, leaving the red the dominant colour. Now what is true of two simple colours is equally true of any number of them, where the rates of absorption differ from one another, and what is true for a solution is true for a transparent solid. In some opaque bodies, such as rocks, the reflected colour often differs slightly from that of the same when they are cut into thin and polished slices, through which the light can pass. The reason is that when opaque, light penetrates to a very small distance through the surface, and is reflected back, whilst in these layers the colour has to struggle through more coloured matter, and emerges of a different hue.
The question why substances transmit some rays and quench others, brings us into the domain of molecular physics. Of all branches of physical science this is perhaps the most fascinating and the most speculative, yet it is one which is being built up on the solid foundations of experiment and mathematics, till it has attained an importance which the questions depending on it fully warrants. We have to picture to ourselves, in the case in point, molecules, and the atoms composing them, of a size which no microscope can bring to view, vibrating in certain definite periods which are similar to the periods of oscillation of the waves of light. At page 26 we have given the lengths of some of the waves which give the sensation of coloured light. Now as light, of whatever colour it may be, is practically transmitted with the same velocity through air which has the same density throughout, it follows that the number of vibrations per second of each ray can be obtained by dividing the velocity of light in any medium by the wave-length. The following table gives roughly the number of vibrations per second of the ether giving rise to the colours fixed by the dark solar lines.

