 полная версия
полная версияColour Measurement and Mixture

Sir William de Wiveleslie Abney
Colour Measurement and Mixture
PREFACE
Some ten years ago there were three measurements of the spectrum which I set myself to carry out; the last two, at all events, involving new methods of experimenting. The three measurements were: (1st) The heating effect; (2nd) the luminosity; and (3rd) the chemical effect on various salts, of the different rays of the spectrum. The task is now completed, and it was in carrying out the second part of it that General Festing, who joined me in the research, and myself were led into a wider study of colour than at first intended, as the apparatus we devised enabled us to carry out experiments which, whilst difficult under ordinary circumstances, became easy to make. On two occasions, at the invitation of the Society of Arts, I have delivered a short course of lectures on the subject of Colour, and naturally I chose to treat it from the point of view of our own methods of experimenting; and these lectures, expanded and modified, form the basis of the present volume.
As a treatise it must necessarily be incomplete, as it scarcely touches on the history of the subject – a part which must always be of deep interest. The solely physiological aspect of colour has also been scarcely dealt with; that part which the physicist can submit to measurement being that which alone was practicable under the circumstances.
W. de W. Abney.South Kensington,
1st May, 1891.
CHAPTER I
Sources of Light – Reflected Light – Reflection from Roughened Surfaces – Colour Constants.
There is nothing, perhaps, in our everyday life which appeals more to the mind than colour, yet so accustomed are the generality of mankind to its influence that but few stop to inquire the "why and wherefore" of its existence, or its cause. To those few, however, there is a source of endless and boundless enjoyment in its study; for in the realms of physical and physiological science there is perhaps no other subject in which experiments give results so fascinating and often so beautiful. Although its serious study must be undertaken with a clear mind, a good eye, and a fair supply of patience, yet a general idea of the subject may be grasped by those who are possessed of but ordinary intelligence.
Colour phenomena are encountered nearly every day of one's life, and the fact that they are so frequently met with, prevents that attention to them, or even their remark. Who amongst us, for instance, has noticed the existence of what are called positive and negative after images, after looking at some strongly illuminated object, or would have gauged the fact that a certain portion of the nervous system can be fatigued by a colour, and give rise to images of its complementary, had not an enterprising advertiser, who manufactures a household necessary, drawn attention to it in a manner that could not be misunderstood.
If on an autumn afternoon we pass through a garden whilst it is still perfectly light, we can notice the gorgeous colouring of the flowers, and appreciate with the eyes the beauty of each tint. As evening comes on the tints darken, the darkest-coloured flowers begin to lose their colour, and only the brightest strike the eye. When night still further closes in every colour goes, though the outlines of the flowers may still be distinguished; and it would not be impossible, in some parts, to see a tiny speck of pale light upon the ground amongst them. This speck of light we should know from experience to be the light from a glow-worm. Why is it that we lose the colour of the flowers and recognize the tiny light from this small worm? The reason for the one is that in order for objects which are not self-luminous to be seen at all, light must fall on them and illuminate them, and the light which they reflect may be coloured if they possess the qualities to reflect coloured light. The glow-worm's light is seen, not because it does not emit light in the day-time, but because the eye, being limited in sensitiveness, is unable to distinguish it when it is flooded with the light of day. The glow-worm, however, is self-luminous, as is shown by the fact that it emits light in the dark, the light itself being slightly coloured if compared with that of day. That a candle-flame or the sun is self-luminous is an axiom, and need not be philosophised upon; but what must be impressed on the reader is, that though an object which requires to be illuminated to be seen, is not self-luminous, yet when illuminated it does in fact become a source of illumination to the eye, although the light is only light reflected from its surface. It is a point worth remembering that the rougher the surface of an object, the brighter to the eye it will be. That is, a coloured object when polished will be a bad secondary source of illumination, as the light incident upon it will be very nearly reflected from the surface, according to the ordinary laws of reflection; but if it be roughened it will become a much better source, as the roughnesses, though obeying the laws of reflection, will reflect light in every direction. A good example of this is an ordinary sheet of glass. Light from a source falling on its surface is scarcely reflected in any direction except in that determined by the ordinary laws of reflection, and it will be scarcely visible to the eye. Grind its surface, however, and the innumerable facets caused by the grinding will reflect light back to the eye in whatever position it be placed, and will thus be distinctly seen.
We may here premise that even the roughest surface will reflect a greater percentage – varying greatly according to the nature of the surface – of light in the direction which it would do if it were a smooth surface than in any other; and in taking measurements of the light irregularly reflected from a rough surface, this fact must be borne in mind.
Not only must we know how colour is produced, but we must also be able to refer it to some standard which shall be readily reproduced, and which shall be unalterable. There are two variable factors which have to be taken into account in colour experiments: the first is the quality of light which illuminates the object, and the second is the sensitiveness of the eye which perceives it, as light is only a sensation which is recognized by the brain through the medium of the eye. We shall, as we go on, see that different qualities of light may cause objects to appear of different hues, and further that eyes may vary in perceptive power, to an extent of which the large majority of people are not aware. Hence it becomes necessary as far as possible to eliminate these variables.
The task which we have set ourselves to perform then, is first to find a suitable light for experimental work, and next to endeavour to refer colour to an eye which has no abnormal defects. This being accomplished, we have then to find means to measure the different constants which are involved in colour, and to refer the measurements to some standard. Colour constants are three, viz. hue, luminosity, and purity; and it will be seen that if these three are determined, the measurement of the colour is complete.
Perhaps the meaning of these terms may require to be explained. The hue of a colour is what in common parlance is often called the colour. Thus we talk of rose, violet, magenta, emerald green, and so on, but for measuring purposes the hue had best be referred to the spectrum colours as a standard (the means of doing so will be shortly explained), for they are simple colours, which can be expressed by numbers. Compound colours, which it may be said are invariably to be found in nature, being mixtures of simple colours, can be just as readily referred to the spectrum. By the luminosity of a colour we mean its brightness, the standard of reference being the brightness of a white surface when illuminated by the same white light. By the purity of a colour we mean its freedom from admixture with white light. An example of different degrees of purity will be found in washes of water-colours of different tenuity. Thus if we wash a sheet of paper with a light tint of carmine, the whiteness of the paper is not obliterated; if we pass another wash over it the whiteness of the paper is lessened, and so on. The lightest tint is that which is most lacking in purity.
CHAPTER II
A Standard Light – Formation of the Spectrum by Prisms and by the Diffraction Grating – Wave-lengths of the principal Fraunhofer Line – Position of Colours in the Spectrum.
As we have to turn to the spectrum for pure and simple colours, from which we may produce any compound colour we may wish to deal with, we will first consider the light with which we shall form it. A spectrum may be produced from any source of light, such as sunlight, limelight, the electric light, gaslight, or incandescence electric light, as also from incandescent vapours, or gases; but it is only a solid which is, or is rendered incandescent, that will give us a continuous spectrum, as it is called, that is, a spectrum which is unbroken by gaps of non-luminosity, or sudden change of brightness, throughout its length.

Fig. 1. – Spectrum of Sunlight.
The great desideratum for the study of colour is a light which not only gives a practically continuous spectrum, but one which is produced by the radiation of matter which is black when cold, and which can be kept at a constantly high temperature. We have purposely said "black" in the sentence above, since it is believed that differently coloured bodies, when heated to equal temperatures, might not give the same relative intensities to the different parts of the spectrum, the variation being dependent on the colour of the heated body. A black body must always give the same visible spectrum when heated to the same temperature. The spectrum of sunlight (Fig. 1) is not continuous, as we find it crossed by an innumerable number of fine lines of varying breadth and blackness. This want of continuity would not be fatal to its adoption were it possible to use it outside the limits of our atmosphere, as then, unless the temperature of the sun itself changed, the spectrum produced would be invariable; but unfortunately the relative brightness or luminosity of the different parts of the spectrum varies from day to day, and hour to hour, according to the height of the sun above the horizon (see Chap. VI.); and its integral brightness varies according to the clearness of the sky. It is evident then, that, as a reference light, sunlight is most unsuitable, so we may dismiss it from our possible standards.

Fig. 2. – The Carbon Poles of an Electric Light.
By the process of elimination we may arrive at the light upon which we can rely, for the purpose we have in view, viz. the production of a spectrum of moderate size, and sufficiently bright to be well viewed when projected upon a screen. For some purposes, as for instance in becoming acquainted with the general character of the spectrum, a feebler light, such as gaslight, or light from electrical glow lamps, may be employed, since the spectrum may be viewed directly by the eye without the intervention of a screen. They have two drawbacks for our object: one being the want of general intensity, and the other the feeble luminosity of blue and violet rays in their spectrum (see page 110). The limelight we can also dismiss for want of steadiness. Its whiteness and luminosity varies according to the oxygen playing on the lime cylinder, rendering the relative intensities of the different parts of the spectrum so erratic as to make it unreliable. This leaves the (electric) arc-light as the only one which is really available. Remember how the arc-light is produced. A current of electricity passes between the ends of two thick black carbon rods, or poles as they are called, through an air space of small interval, and the passage of the current renders the tips of these rods white-hot (Fig. 2). The centre of the end of one pole, called the positive pole, where a crater-like depression is formed, is the part which attains the whitest heat, and its temperature seems to be constant, and to be that of the volatilization of carbon. Numerous experiments have been made by the writer, and he has found that the light emitted by this crater in the positive pole is, within the limits of the error of observation, always of the same whiteness, and consequently gives a spectrum which is unvarying in the proportionate intensities of the different colours. When the experiments made to determine the luminosity of the spectrum are described, the method of ascertaining this will be readily understood.
In the spectrum produced by this light there are two places in the violet where there are bands of violet lines slightly brighter than the general spectrum. They are principally due to the light emitted from the incandescent vapour of carbon, which is volatilized and plays between the two poles (see Fig. 2); but as these bands are of but small visual intensity, and situated towards the limit of the visible spectrum, they do not interfere with eye-measures of colours, though they do, to a certain extent, to the analysis of radiation by photography. If we throw the positive pole a little behind the negative pole we can, however, considerably mitigate this evil. We can separate the carbon rods to such a degree that the white-hot crater faces the observer, and a good deal of the arc is hidden. This is well seen in the figure.
We have now described the light we have adopted, and the reasons for adopting it; and having obtained our light, we can now consider by what plan we shall form our spectrum. There are two ways open to us – one by glass prisms, and the other by a diffraction grating. Glass prisms separate white light, or indeed any light, into its components, from the fact that the refraction of each coloured ray differs from every other. Thus the red rays are least refracted, and the violet the most, and the yellow, green and blue are intermediate between them, being placed in the order of least refrangibility. Between these there is of course every shade of simple colour, one melting into the other. In order to form a pure and bright spectrum with prisms, in a room of limited dimensions, we have to use certain auxiliary apparatus which are not positively essential, though convenient. The real essentials to form a spectrum are a narrow slit, a glass prism, with perfectly plane faces, and a lens. If this be the only apparatus available, the slit must be placed at a long distance from the prism, the beam of light must pass through the slit on to the prism, and the lens must be placed at such a distance from the slit that it forms a sharp image on a screen. When the light passes through the prism, the screen will have to be rotated in the arc of a circle, so that its distance from the slit measured along the line of the ray to the prism, and from the prism to the screen, is the same as it would be without the intervening prism. An apparatus of this description is not convenient, however, as it requires much more space than is often available. If a lens be placed between the slit and the prism, at exactly its focal length from the former, the light entering the slit will, after passage through the lens, emerge as parallel rays, that is, they will emerge as they would do if the slit were placed at an infinite distance from the observer.
The focal length of this collimating lens need not be greater than twelve to eighteen inches, so that the great space required by the cruder apparatus is very much curtailed. The lens and slit are mounted one at each end of a tube of the necessary length, and are thus handy to use.
Instead of one prism two or three may be used, giving an angular dispersion of the spectrum two or three times respectively greater than that which would be given by only one prism; consequently to obtain a given length of spectrum with the increased dispersion, the focal length of the lens used to focus the image on the screen may be diminished.
The drawback to the use of prisms is that the dispersion of the red end of the spectrum is much less than that of the blue end, and is apt to give a false impression as to the relative luminosities of, and length of spectrum occupied by, the different colours. In some text-books it is told us that the diffraction grating gives us a dispersion which is in exact relation to the wave-length. This is not true, however, as it can only give one small portion in such relationship, and that only when it is specially set for the purpose. The subject of diffraction is one into which it would be foreign to our purpose to wander. We may say that for measures such as we shall make, it is handier to employ prisms, as the prismatic spectrum is more intense than the diffraction spectrum. This can be readily understood when we consider the subject even superficially. If we throw a beam of light on a grating which contains perhaps some 14,000 parallel lines in the space of one inch in width, the lines being ruled on a plane and bright metallic surface, and receive the reflected beam on a screen, the appearance that is presented is a white central spot, together with six or seven spectra of gradually diminishing brightness on each side of it, all except the first pair overlapping one another. That these different spectra do exist can be readily shown by placing in the beam a piece of red glass, when symmetrical pairs of the red part of the spectrum will be found, one of each pair being on opposite sides of what will now be the central red spot. Half the light falling on the grating is concentrated in this central spot, and the remaining half goes to form the spectra; the pair nearest the central spot being the brightest. We thus are drawn to the conclusion that at the outside we can only have less than one-quarter of the incident light to form the brightest spectrum we can use. With two good prisms we use at last three-fourths of the incident light, so that for the same length of spectrum we can get at least three times the average brightness that we should get were we to employ a diffraction grating.
We must now refresh the reader's memory with a few simple facts about light, in order that our meaning may be clear when we speak of rays of different wave-lengths. Every colour in the spectrum has a different wave-length, and it is owing to this difference in wave-length that we are able to separate them by refraction, or diffraction, and to isolate them. Light, or indeed any radiation, is caused by a rhythmic oscillation of the impalpable medium which we, for want of a better term, call ether, and the distance between two of these waves which are in the same phase is called the wave-length of the particular radiation. The extent of the oscillation is called the amplitude, which when squared is in effect a measure of the intensity of the radiation. Thus at sea the distance between the crests of two waves is the wave-length, and the height from trough to crest the amplitude; and the intensity, or power of doing work, of two waves of the same wave-lengths but of different heights, is as the square of their heights. Thus, if the height of one were one unit, and of the other two units, the latter could do four times more work than the former. The waves of radiation which give the sensation of colour in the spectrum vary in length, not perhaps to the extent that might be imagined, considering the great difference that is perceived by the eye, but still they are markedly different. The fact that the spectrum of sunlight is not continuous, but is broken up by innumerable fine lines, has already been alluded to. The position of these lines is always the same, as regards the colour in which they are situated, and is absolutely fixed directly we know their wave-length; hence if we know the wave-lengths of these lines, we can refer the colour in which they lie to them. Now some lines of the solar-spectrum are blacker and consequently more marked than others, and instead of referring the colours to the finer lines, we can refer them to the distance they are from one or more of these darker lines, where these latter are absolutely fixed; in fact they act as mile-stones on a road.
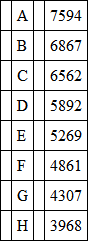
In the red we have three lines in the solar spectrum, which for sake of easy reference are called A, B and C; in the orange we have a line called D, in the green a line called E, in the blue F, in the violet G, and in the extreme violet H. These lines are our fiducial lines, and all colours can be referred to them. The following are the wave-lengths of these lines, on the scale of 1/10,000,000 of a millimetre as a unit
When the spectrum is produced by prisms the intervals between these lines are not proportional to the wave-lengths, and consequently if we measure the distance of a ray in the spectrum from two of these lines, we have to resort to calculation, or to a graphically drawn curve, to ascertain its wave-length. For the purpose of experiments in colour the graphic curve from which the wave-length can immediately be read off is sufficient. The following diagram (Fig. 3) shows how this can be done.
The names and range of the principal colours which are seen in the spectrum has been a matter of some controversy. Professor Rood has, however, made observations which may be accepted as correct with a moderately bright spectrum. If the spectrum be divided into 1000 parts between A in the red, and H, the limit of the violet, he makes the following table of colours.


Fig. 3. – Curve for converting the Prismatic Spectrum into Wave-lengths.
In the above scale (Fig. 3) A = 0, B = 74·0, C = 112·7, D = 220·3, E = 363·1, F = 493·2, G = 753·6, H = 1000.
These are the main subdivisions of colour, but it must be recollected that one melts into the other. When the spectrum is very bright the colours tend to alter in hue; thus the orange becomes paler, and the yellow whiter, and the blue paler. On the other hand, if the spectrum be diminished in brightness the tendency is for the colours to change in the opposite direction. Thus the yellow almost disappears and becomes of a green hue, whilst the orange becomes redder, and the spectrum itself becomes shorter to the eye than before.
Let us strictly guard ourselves, however, from the criticism that all eyes see not alike. Suffice it to say that the above table is correct for the ordinary or normal eye, and does not necessarily apply to those who have defective vision as regards colour sensation.
CHAPTER III
The Visible and Invisible Parts of the Spectrum – Methods for showing the Existence of the Invisible Portions – Phosphorescence – Photography of the Dark Rays – Thermo-Electric Currents.
We are apt to forget, when looking at the spectrum, that what the eye sees is not all that is to be found in the prismatic analysis of light. The spectrum, it must be recollected, is not limited to those rays which the eye perceives. There are rays both beyond the extreme violet and below the extreme red, which exist and which exercise a marked effect on the world's economy. Thus, rays beyond the violet are those which with the violet and the blue rays principally affect vegetation, enabling certain chemical changes to take place which are necessary for its growth and health; whilst the rays below the red are those possessing the greatest amount of energy, and if they fall upon bodies which absorb them, as very nearly all bodies do to a certain extent, they heat them. The warmth we feel from sunlight is principally due to the dark rays which lie below the red of the spectrum.
The existence of both kinds of these dark rays may be demonstrated in a very simple manner by the effect that they produce on certain bodies. For instance, there is a yellow dye with which cheap ribbon is dyed, which if placed in the spectrum and beyond the violet causes a visible prolongation of the spectrum. The light in the newly-seen and once invisible part of the spectrum is yellow, the colour of the ribbon itself. In fact, the whole of that part of the spectrum, which on the white screen is seen as blue and violet, becomes yellow, the red and green remaining unchanged. This change in colour is due to fluorescence, a phenomenon of light which Sir G. Stokes found was caused by an alteration in the lengths of the waves of light when reflected from certain bodies. It is not meant to imply by this that the wave-length of any ray falling on a body can be altered by reflection, but only that the body itself on which the rays fall emits rays of light which are not of the same wave-length as those which fall upon it. Now it is a fact that the rays that lie beyond the violet, and which are ordinarily invisible, are shorter than the violet rays, and that these are shorter than the yellow rays. It follows therefore that when, what we may now call, the ultra-violet rays fall on the yellow dyed ribbon, the waves emitted by it are so lengthened that they appear yellow to the eye instead of dark, violet, or blue.

