
Полная версия
Collins New Naturalist Library
Some more recent cave biologists have noticed that captive Olms regularly slough and then eat the mucus layer which, like an extra skin, covers and protects their whole body. Mucus is sticky and microscopic examination has shown that in captive amphibians it becomes encrusted with bacteria, algae and protozoa. So de Kerville’s amazing ‘non-feeding Olm’ may all the time have been sneaking clandestine meals of diatoms-in-slime – not the tastiest of fare, but enough to keep it ticking over. Streams sinking into caves must carry with them a fair load of phytoplankton, and it may be that the Olm’s mucus-eating behaviour in captivity has some adaptive significance in the subterranean rivers where it lives.
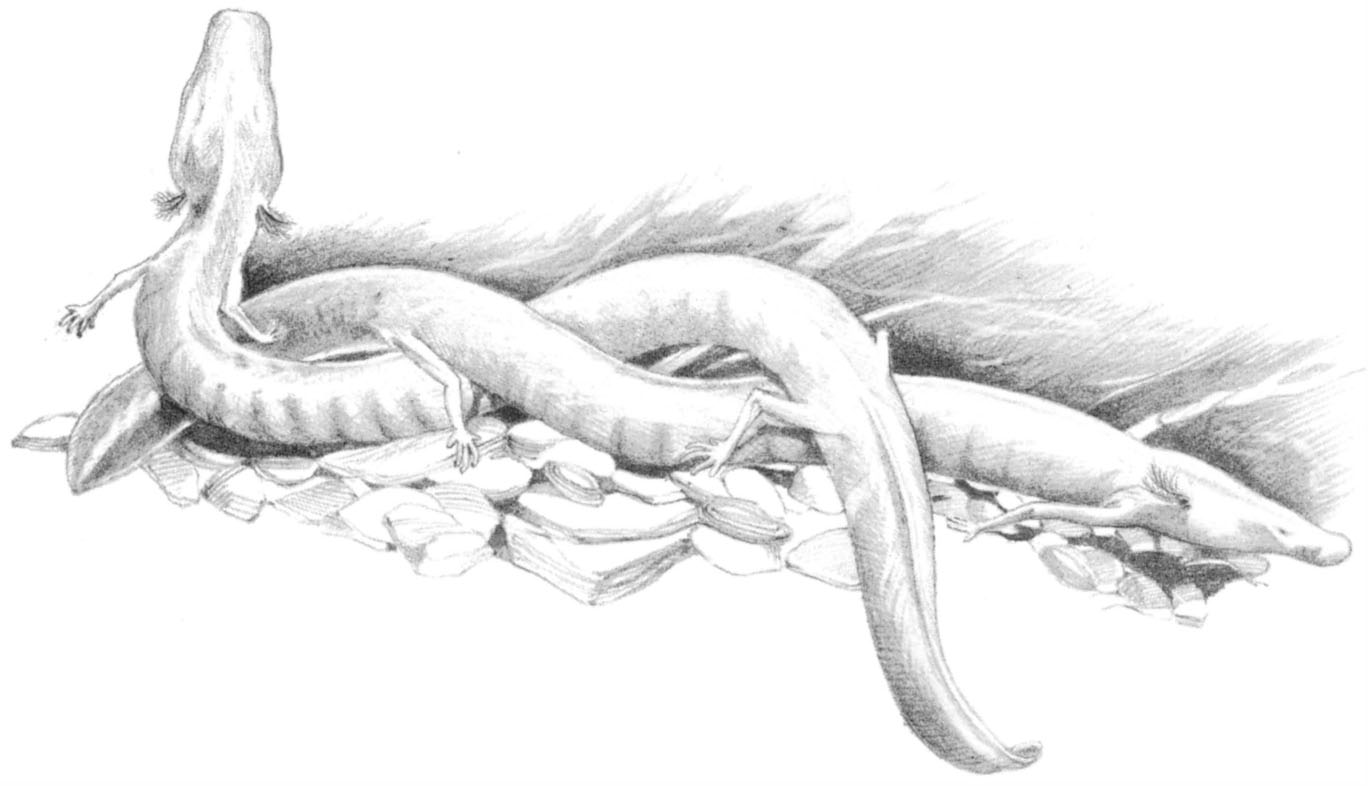
Fig. 2.8 Olms (Proteus anguinus) – blind, depigmented cave salamanders which retain gills and an aquatic lifestyle as adults.
In a closed system, such as our Olm aquarium, the recycling of dissolved nutrients could go on endlessly, providing the Olm did not lock them up as extra Olm tissue. Once equipped with a source of energy – sunlight from the nearest window will do – the tank becomes a self-perpetuating ‘mini-ecosystem’. Because caves are perpetually dark, production cannot meet demand and they can therefore never be considered as proper self-sustaining ecosystems. But the waters which form, enlarge, infill and eventually destroy caves almost invariably carry organic compounds – complex chemicals gathered from the soil or the breakdown of animal and plant detritus. Organic chemicals (dissolved in water, or clumped together in big lumps of detritus) fill the role of an energy source in the cave. There has been much speculation by cave biologists over the extent to which dissolved organic substances can be absorbed directly by aquatic cavernicoles. So far the evidence is inconclusive, but there is no doubt that they are captured by microfungi, bacteria and protozoa, which are plentiful at least in some allogenic cave waters. So, one way or another, all organic material entering the cave becomes available to the bottom rung of cave animals – the detritivores which fill the equivalent role to the primary consumers of the sunlit world above.
While the photosynthesising parts of plants have no place in caves, there is no reason why their roots should not penetrate into subterranean voids. Indeed, in the lava tubes which run just beneath the skin of the active volcanoes of Hawaii, tree roots form dense subterranean forests which support a range of sap-sucking bugs, root-chewing caterpillars and their predators; pale-skinned, eyeless relatives of species found in the forest above. Roots seldom penetrate into macrocaverns in Britain, but they may constitute a significant food source in a number of other cave habitats, such as the SUC, culverts and slutch caves. No one seems to have investigated root-associated faunas in such locations, but it would surprise me if such a widespread niche is not occupied by at least one insect specialist.
Second-hand plant material takes many forms: autumn leaves carried on a sinking stream, tree trunks hurled into a pothole by violent floods, or well-rotted humus quietly inched down the cracks of a limestone pavement. Usually by the time such materials reach the depths of the cave where the true cave faunas live, they have been thoroughly pulverised by water and rock, tenderised by bacteria and fungi, and often enough, passed through a series of invertebrate guts.
This was brought home to me during my first visit into the higher levels of GB Cave in the Mendip Hills, several years ago. The upper levels of the cave contain a series of narrow rifts which leak water from the overlying land whenever it rains. I was hunting for tiny cave beasts in these upper levels, and looking out for patches of fresh mud which I guessed would be organically richer and so would contain more life. I soon began to realize that each and every sediment cone emanating from each and every leaky crack was made up of thousands of tiny mud pellets, like scaled-down grains of rice. They were arthropod droppings – hundreds of millions of them, forming a deposit which over the centuries was gradually filling up the upper dry passages, and probably the lower reaches beneath the water table too. Fortunately, most cavers are blissfully unaware of the true nature of the substance which they spend each weekend crawling through and, no doubt, liberally ingesting themselves.
Biologists used to studying ecosystems based on living plants too often treat detritivores – animals which eat dead organic material – as a single dietary category. In the cave ecosystem, all the first-level consumers are ‘detritivores’ and it is surprising just how many different specializations they manage. This is most clearly seen in guano communities, where chains of very specialized organisms use different components of the insect remains which form the bulk of bat droppings. Stuart Hill has studied the ecology of one such community in a bat cave in Trinidad. Guano of the funnel-eared bat, Natalus tumidirostris (notorious, incidentally, as a carrier of the human diseases, relapsing fever and blastomycosis), was eaten as soon as it fell by a cockroach, Eublabarus distanti, which removed most of its fat and some of its protein (much of the unbound protein having been already stripped out in the gut of the bat). The cockroach droppings, still rich in chitin, were decomposed by a fungus, Penicillium janthinellum. This in turn was fed on by the mite Rostrozetes foveolatus and various other tiny arthropods. The mites in turn were eaten by other arachnid predators. Similar sorts of food chains probably operate in British bat caves, but the research has not yet been done.
In our present ignorance, we really do not know exactly what many of our British cave detritivores get out of their ‘junk food’ diets. There must be some degree of specialization, because certain types of materials are largely ignored by certain cave animals, while others attract them strongly.
One way to study dietary specialization is to put down baits in the cave and see what species come to them. The baits should be very small or they will provide an un-natural boost to the numbers of certain species and so upset the balance of the cave community.
I tried a baiting programme in the Ogof Ffynnon Ddu system in Wales, during the course of a wider survey of the fauna. I already knew, from a number of earlier collecting visits, that at least six species of fly were common in the upper level passages (a dung fly, a coffin fly, a winter gnat, and three or more fungus gnats), together with eleven springtail species, a millipede, a woodlouse and various beetles and mites. All were associated with detritus in one form or another, so when I put down small pieces of raw liver or cheese in various choice spots, I expected to gather quite a collection of invertebrates. Over the next couple of weeks I came back regularly to check the baits and was disappointed to find nothing on them. By now the liver was beginning to get a bit unsavoury and the cheese had gone slimy. Two and a half weeks after placement, I found every single liver and cheese bait crawling with the slender maggots of the winter gnat, Trichocera maculipennis, but still nothing else came near them. Eventually, the larvae crawled away to pupate, the bacterial smell subsided, and only then did a few Folsomia springtails gather in the area. So, far from being unspecialised scavengers, these detritivores showed themselves to be quite a discerning bunch.
One of the key factors in determining how the cave fauna will respond to a particular source of detritus seems to be whether it is first attacked by fungi or bacteria. Many fungi exclude bacteria from their chosen food by secreting antibiotics, and avoid other foods of the wrong pH which are decomposed by bacteria. Sometimes the dominance of either one or the other is determined by the size of the potential food source. In the course of a study of lava cave invertebrates on Kilauea volcano in Hawaii, I put out baits of supermarket white bread, to see what would be attracted. Some baits were in the form of crumbs spread along the rough cave wall, others were big chunks which I kneaded into golf-ball-sized lumps. The crumbs quickly went soggy and grew a thin gelatinous slime of yeast-like fungi which attracted cave-specialised millipedes and crickets; while the chunks became bacterial stink bombs infested by scuttle-fly maggots, but shunned by all the true cave fauna.
Several European biospeleologists have noticed a similar specialization between bacterial- and fungal-feeders. In general it seems that ‘surface grazing’ arthropods, such as millipedes, isopods and springtails, tend to be fungus-eaters; while ‘gulpers’ and burrowers, such as fly larvae and earthworms tend to be bacterial feeders. However, even closely related species within the same group can specialize to different foods to avoid competition. An example is found in the springtails Tomocerus minor and its congener T. problematicus, which co-exist in similar habitats in the Grotte de Sainte-Catherine, in the Ariège region of France. The former mainly munches fungi, while the latter feeds largely on bacteria-rich silt.
In general it seems that microfungi, which occur on a variety of substrates, including wood, animal faeces and plant detritus, form the most important dietary component for most terrestrial cavernicoles. In a study of several Virginia caves, Dickson and Kirk (1976) found that the abundance of the cavernicolous invertebrates was correlated with the abundance of microfungi and with high fungal-bacterial ratios, but not with abundance of bacteria or actinomycetes.
Friedrich, Smart and Hobbs (1982) summarized the literature on bacterial counts for cave sediments and waters. As might be expected, heterotrophic bacteria (which feed on inwashed organic material) are far more numerous than autotrophic bacteria (which utilise the oxidation of inorganic compounds) – but there are very wide variations. Sediments give consistently higher bacterial counts, generally of the order of 10 x 106 g-1, but with large variations either side; while cave waters give very much lower counts, generally of the order of 100 ml-1. These authors’ own figures for different types of water inputs in Mendip caves are interesting. Swallets feeding directly into open cave passages give the highest figures (500 ml-1). Percolation recharge which is integrated into mesocavernous fissures and conduits has an intermediate bacterial count (50–300 ml-1), while diffuse flow waters in phreatic microcaverns (sampled via boreholes) give a very low count (2 ml-1). One of their conclusions is that water flow through the limestone aquifers feeding the major springs used as drinking supplies around Mendip does not provide any significant filtration of microbial input. While bacterial counts at the major Mendip risings in general do not give cause for public health concern, this may not be the case in other springs, particularly in tropical countries, used as an untreated drinking supply on the supposition that water which bubbles out of the ground must be pure. Sinkholes are widely used as rubbish dumps, attracting disease-carrying rodents, and the water which enters them may reappear many miles away as a spring, perhaps in a different valley. The late Dr Oliver Lloyd, a well-known Mendip caver, contracted Weil’s Disease from Leptospira bacteria present in Longwood Cave on Mendip, while several members of expeditions to Borneo in 1980 and 1984 (myself included) contracted a similar form of leptospirosis in the famous Mulu caves, and nearly died as a result.
In slow-moving underground streams with mud bottoms, and in drip- and seep-fed pools isolated from swallet-fed streamways, microfungi are more common than bacteria and are correlated with the abundance of macroscopic cavernicoles. Microfungi and bacteria may be utilized directly by such cavernicoles, or may be eaten by Protozoa, nematodes and other micro-fauna which are in turn eaten by Crustacea (isopods and amphipods) and aquatic insect larvae.
I have tried so far to paint a picture of the cave community as a fairly structured world, where, despite the absence of green plants, the lowly animals at the bottom of the food chain still manage a fair degree of dietary specialisation, preferring some kinds of detritus or living prey over others. In fact, the degree of specialization may be even more pronounced: not just according to the nature of the food source, but even according to the size chunks in which it is packaged. Thomas Poulson and Tom Kane, working in the enormously complex Mammoth Cave system in Kentucky, have found that the terrestrial arthropods in the cave use energy availability as the main basis for dietary specialization. Of the 44 species regularly recorded in their study area, 30 were primarily associated with one food, seven with two, six with three, and two with four of the six natural foods available to them. All the species which used more than one food source picked different stages in the decomposition of each of their foods, or different places along the gradient of food concentration so that they fed at a unique level of energy availability. Two millipedes illustrate how this works. Scototerpes feeds on the scattered droppings of cave crickets and to a lesser extent on very dispersed leaf litter in the last stages of decomposition and on veneers of leached organic matter deposited by floods on the cave ceiling and walls. The other common cave millipede, Antriadesmus specializes on cricket droppings in areas where they form thick deposits. Not surprisingly, the latter species turns out to have a higher metabolic rate and to lay more eggs than its more frugal cousin.
Poulson and Kane’s finding may go some way to explaining the unattractiveness of my cheese and liver baits in OFD. It could be that unusual concentrations of protein-rich food entering an oligotrophic (food-poor) cave, may be shunned by the cave community (except guanobia) as they represent a level of energy availability well above that to which the cavernicoles are adapted. But there may be a simpler explanation. Kathleen Lavoie of the University of Illinois noted that certain cave fungi grew better on large droppings (such as those of pack rats) than on those of smaller species. Rapid colonization by fungal hyphae formed a meshwork barrier which seemed to exclude cave arthropods from this otherwise tasty food resource. So, far from cave arthropods shunning large items of food, it may be that other, more opportunistic forms of life actively keep them at bay.
So far, we have considered only detritus as a food-source. While in sheer numbers, detritus-eaters must of course form the bulk of the cave community, it is predators and omnivores which have the greatest scope for dietary specialization, providing they are able to survive at low population densities. The predators in British caves are unspectacular creatures: mites, spiders, beetles and their larvae, amphipods, caddis larvae and flatworms. Once again, we can only speculate as to the degree of ecological specialization which they enjoy – they have not yet received any detailed study in Britain. The Americans, however, have studied their own equivalent species in considerable detail and, as there is no reason to expect cave predators of temperate American caves to behave very differently from our own, their research findings are worth repeating here.
Tom Barr studied six cave-evolved ground beetles which co-exist in Mammoth Cave. One of them, Neaphaenops tellkampfi, feeds almost exclusively on the eggs of cave crickets. It finds them by smell, digs them out of the sand in which they were laid, then punctures them with specially-elongated mandibles and sucks out the contents. The other five beetles are all closely related in the genus Pseudanophthalmus, and all feed on different prey or in different microhabitats. Even the two near-identical-looking species P. menetriesii and P. striatus feed quite differently: one hunts for small arthropods in rotting wood, the other digs for tubificid worms in cave silt. Our own cavernicolous carabid, Trechus micros, feeds in a similar way. I have watched it work over a tidal mud bank in Otter Hole in the forest of Dean, pocking the surface with hundreds of tiny pits, as it thrust its head repeatedly into the soft sediment in search of prey.
The tidal mud of Otter Hole is an unusual cave sediment which supports an exceptional fauna. Estuarine muds of the Bristol Channel have been estimated to have a productivity four times that of good arable soil. They are rich in organic material and correspondingly smelly. The mud is kept sloshing up and down the upper reaches of the estuary by fierce tides which have an amplitude here of 13 m, one of the greatest tidal ranges anywhere on our side of the Atlantic. During the highest Spring tides, when the cave stream is itself swollen by rains, it is usual for the entrance passages of the cave to flood right up to the roof and to stay flooded for several weeks. Cavers know this, and avoid the cave during this danger period. Eventually the trapped waters drain slowly away, but in the meantime their sediment load has settled out as a rich brown deposit which coats the walls and ceiling of the cave right up to the high-tide mark. The new mud is soon invaded by a wealth of invertebrates, including a millipede, Brachychaetuma melanops, and a rove beetle, Aloconota subgrandis, found in no other British caves. The richness of the cave fauna is a direct result of the floods.
The importance of seasonal flooding to cave invertebrates has been noted by many biospeleologists. In the food-poor alpine caves of the Pyrenees and Spanish Cantabrians, almost the whole fauna lives in the ‘intertidal zone’ of flood-prone passages. In the enclosed space of a cave, flooding can take one of two forms: ‘flash floods’ and ‘ponding’. In the former, a sudden rush of water temporarily approaches the carrying capacity of a streamway, sweeping all before it. This is the type of flood dreaded by cavers, and passages which are prone to such events are rigorously avoided whenever there is a risk of heavy rain in the catchment area. ‘Ponding’ floods happen more gently. They usually occur where collapsed blocks or sediment impede the flow of escaping water, so that any slight increase of input causes a temporary pond or lake to form behind the obstruction. The water may have been flowing quite quickly up to the barrier, but now it slows, depositing its sediment load. As the waters recede, the local invertebrates rush out of hiding to ‘beachcomb’ for the juciest morsels.
In temperate caves, flooding is a strongly seasonal, and therefore predictable, phenomenon – and many cave species time their cycles of activity and reproduction around it. The advantage of synchronized breeding is obvious in cave species which occur at low densities and in which only a small proportion of females are capable of reproduction in a given year. Tom Poulson, of Yale University, has made a special study of aquatic cave communities in the USA. He explains the complex relationship between cavernicoles and floods as follows:
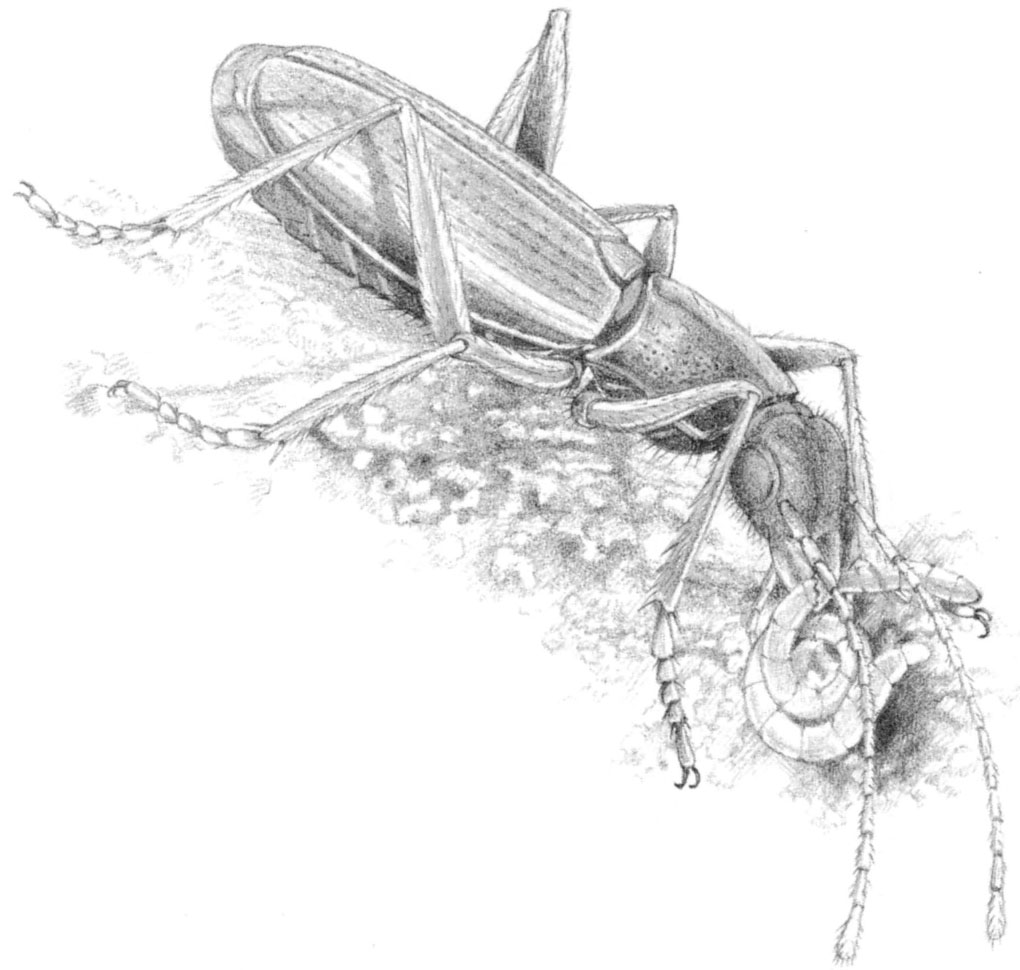
Fig. 2.9 The ground beetle Trechus micros digging an enchytraeid worm from the silt of Otter Hole in the Wye Valley.
“Annual growth and breeding cycles in caves are cued by spring floods, specifically by changes in temperature, food supply, amounts of solute, turbidity and current … Scale growth rings of amblyopsid cave fish, and probably other cave fish, form during floods while plankton and organic matter are being replenished, but the fish are secretive, inactive and not feeding. The amblyopsid cave fish Chologaster agassizi, Typhlichthys, Amblyopsis spelaea and A. rosae breed in spring towards the end of the yearly floods or high water, and the young appear during the period of low water 3 to 8 months later when residual food is still present and chances of injury from floods are low. … Some snails, isopods, amphipods and decapods also breed in spring and early summer … [for example] breeding in the shrimp Paleomonetes ganteri and the crayfish Orconectes pellucidus precedes high water, with maximum organic inwash, by 4 to 6 months.”
James Keith has found a clear seasonal reproductive cycle in the American cave beetle Pseudanophthalmus tenuis, which inhabits flood-prone mud banks in Murray Spring Cave, Indiana. Across the Pacific, Chris Pugsley has studied the New Zealand glow-worm Arachnocampa luminosa whose starry displays form the centre-piece of a tourist development at the famous Glow-worm Grotto at Waitomo. Although most stages are present throughout the year, numbers of larvae (the feeding stage) show a clear peak in late spring / early summer, when their food (winged imagines of aquatic insect larvae) are abundant, and the evaporation rate in the cave is at its lowest level.
Seasonal variations in food supply in caves are not due solely to flooding. Bats have a predictable seasonal occurence in temperate caves, and this might be expected to strongly affect members of the guano communities which depend on their presence. Harris (1970) has described the cycle associated with occupation of a cave by a maternity colony of Bent-winged Bats in Australia. As the bats arrive, there are fast changes in food, temperature, moisture relations and pH. Food quantity, rate of daily input and freshness all increase. The guano temperature rises 10°C within a week. The fresh guano, along with bat urination combine to increase the relative humidity from 60 to 95%, and the substrate becomes visibly moist. The urine-ammonia aerosols and faecal material modify the substrate pH and other chemical characteristics. How guanobious cavernicoles respond to such changes does not seem to have been studied in any detail, but it is known that population levels of many species of guanobia increase sharply when fresh guano becomes available, and decrease when it is not.
Microclimate
Until a few years ago, I had often wondered how the ‘terrestrial’ inhabitants of flood-prone passages and mesocavern cracks survived the regular immersions on which their livelihoods depend. It took a visit to the New Guinea highlands to reveal the secret. I was involved, with a large British expedition, in exploring the huge labyrinth of Selminum Tem – then the longest cave in the southern hemisphere. One day, while a small group of us were in the bowels of the system, the heavens opened and 10 cm of rain fell in a couple of hours. The cave streams rose by several feet in a matter of minutes, and we were lucky to get out in one piece. Two of the team were working in a young, immature network of passages deep below the main trunk of the cave and in their haste to escape the rising water, they dropped a quantity of expensive equipment. So a couple of days later, a colleague and I returned to retrieve it. The passage had obviously flooded to the roof, and the water level was still falling, amid distant gloops and gurgles. The walls and ceiling of the passage were coated with a thin layer of wet black mud, spangled here and there with fragments of soggy biscuit, washed from a packet dropped by the fleeing cavers two days previously. Several of the fragments had already attracted beetles and millipedes, and as I watched, a glistening wet millipede slowly emerged from the depths of a crack and headed across the mud in the direction of the nearest biscuit fragment. Further along the passage, millipedes of two different species appeared to be feeding on the floor of a temporary puddle – underwater. Later I watched a woodlouse doing the same thing. It seemed that the cave community here was quite amphibious; sheltering in cracks and crevices as the waters swept through their home and sallying forth to feed once the flood had passed by.
Конец ознакомительного фрагмента.
Текст предоставлен ООО «ЛитРес».
Прочитайте эту книгу целиком, купив полную легальную версию на ЛитРес.



