Полная версия
Pediatric stroke. Revascularization and reconstructive surgery in children with cerebrovascular disease
3. pathologic looped or spiral tortuosity as well as knotting (up to 360°);
4. combination of various deformations [12].
Presently, the modified J. Weibel – W. Fields and H. Metz classification is actively used:
1. Tortuosities: C- and S-shaped elongation of the ICA or deformation along the ICA course (Fig. 3A, B);
2. Insignificant deformation: angulation or kink between two ICA segments with a loop formed at an angle exceeding or equal to 60°, causing a local stenosis of a major artery (Fig. 2A);
3. Moderate deformation: angulation or kink between two ICA segments with a loop formed at an acute angle equal to 30—60°, causing a local stenosis of a major artery (Fig. 2B);
4. Marked deformation: angulation or kink between two ICA segments with a loop formed at an acute angle less than 30°, causing a local stenosis of a major artery (Fig. 2C);
5. Looping or knotting: excessively long ICA forming a marked S-shaped tortuosity or an annular configuration, where more than two ICA segments lying on different planes are involved in the process (Fig. 3C) [264].
Another important property of a deformation is its hemodynamic significance determined by the growth degree of the linear blood flow rate (LBFR) in the deformation area due to its local contraction, thus reflecting the intensity of septal stenoses and twist of the artery without distal hypoplasia signs and decrease of volumetric blood flow in the deformed artery. The tortuosity is considered hemodynamically significant, when the increasing LBFR in the deformation area exceeds 170 cm/s (moderate significance). If the LBFR exceeds 250 cm/s, it is considered as evident hemodynamic significance, and when it exceeds 300—350 cm/s with the presence of a turbulent noise – as rough [26].
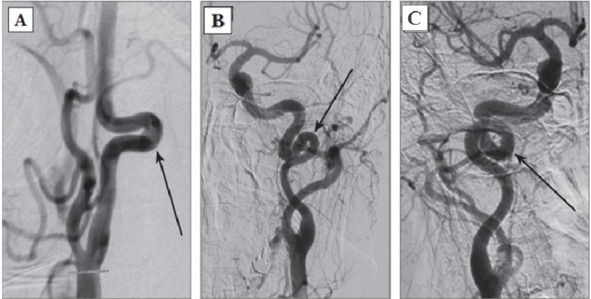
Fig. 3: Types of pathological deformations based on CAG: A – C-shaped tortuosity of ICA (arrow indicated); B – S-shaped tortuosity of ICA (arrow indicated); C – ICA loop (arrow indicated).
According to literature data, a forward course of major vessels is noted in 65—70% of persons, vascular deformations – in 23—40%, including the pathological, hemodynamically significant tortuosities – about 9—16% of cases. According to data of J. Weibel and W. Fields (1965), C- or S-shaped course of a vessel elongated approximately by 4 cm occurs twice more often unilaterally than bilaterally. Other types of pathological deformations are revealed equally often, regardless of the gender and the age, while unilateral pathological deformations occur also twice more often than bilateral [14; 20]. Hemodynamically significant, pathological tortuosities cause both acute cerebrovascular diseases and chronic cerebrovascular insufficiency. While a child grows, the pathological tortuosity of the ICA may be leveled completely or the artery may get «straightened», which is accompanied by a recovery or an improvement of the blood flow and the regression of neurologic disturbances [14].
Moyamoya is a rare disease [208] characterized by a progressive spontaneous stenosis or occlusion of a supraclinoid segment of an ICA (single or both) at the level of the siphon and the initial segments of the anterior cerebral artery (ACA) and the middle cerebral artery (MCA) with the subsequent involvement of the VBS. The specific feature of this disease is the secondary formation of a basilar, anastomotic capillary network, resembling a small cloud of smoke (Fig. 4) during the angiographic imaging, which is pronounced in Japanese as «moyamoya». This word has become an official name of the disease. In 40% of cases in the moyamoya disease a bilateral impairment of the ICA is noted; initially the ICA is involved only on one side [233; 285]. The «moyamoya syndrome» term is more often used for angiographic description of the pathology [266].
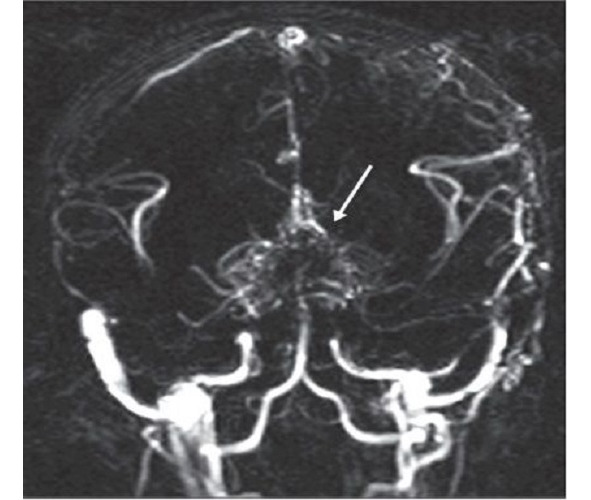
Fig. 4: MRA of a patient with the moyamoya disease. The secondary formation of anastomotic network resembling a cloud of smoke is indicated with an arrow.
According to data from various sources, the «moyamoya» term was first used by A. Takeuchi and J. Suzuki in 1969 [254]. According to other data, the discovery of this disease is related to an earlier period, when in 1937 K. Shimizu implemented the carotid angiography technique in Japan [205]. Shortly after the World War II, neurosurgeons started to apply this type of test actively, which permitted to diagnose and to study the moyamoya disease. It was found then that this pathology can be often seen in the countries of South-East Asia. The research works by A. Takeuchi, K. Shimizu (1957), N. Moriyasu (1964), T. Kudo (1968), J. Suzuki and A. Takaku (1969) have contributed a lot into the worldwide awareness and study of the disease.
It was shown that the highest morbidity rate of the moyamoya disease in the world is noted in Japan – 4—5 incidents per 100,000 population annually. By way of contrast, in 2005 in America the morbidity rate of the moyamoya disease was 0.086 incidents per 100,000 population [266]. R. Smith and J. Scott (2012) claim that in America the moyamoya disease rarely occurs in children – 1 incident per 1,000,000 – and becomes the cause of 6% of all pediatric strokes [243]. According to data of various European clinics, for the last 5—6 years the number of patients, especially children, with the moyamoya disease has increased in Europe, and it still continues growing [139]. Such a tendency might be connected with the improved diagnostics of cerebrovascular diseases, although this issue is studied little. Some prevalence of the morbidity is noticed in women (the ratio of female and male patients is 1.6:1). I. Ahn et al. (Korea, 2014) analyzed the statistical data for the period from 2007 till 2011. In 2011 the total number of patients with the moyamoya disease was 8,154 in Korea, during the period from 2007 till 2011 the morbidity was recorded on the level of 2.3 per 100,000 people, while in 2011 it was 16.1 per 100,000 people; the ratio of female and male patients was 1.8:1 [41].
In Russia there are singular publications about this disease [15; 18; 29].
The moyamoya disease has two age-related peaks of clinical manifestation: the first one comes on children at the age of 5—10 years old, while the second one – on the age of 30—40 years old [258; 266]. The pathologic process is most active approximately until the age of 10 years, and it gets stabilized approximately by the age of 20 years [208; 270].
The etiology of this disease is actively discussed, although it still remains unknown [208]. It is supposed that the moyamoya disease may be either congenital or acquired [133]. It is noticed to be associated with other systemic and non-systemic diseases (Down’s syndrome, neurofibromatosis type I, autoimmune diseases, tuberous sclerosis, atherosclerosis, fibro-muscular dysplasia, thalassemia and sickle-cell anemia, thyroid disorders) as well as with radiation therapy of basilar gliomas in children with a craniocerebral injury [135; 175; 224; 234; 270].
Hereditary factors play an important part in the moyamoya disease [196; 277]. There are some known familial cases of the disease. According to some data, this disease has a familial nature approximately in 15% of patients. Due to this, over the latest years the attempts have been made to find a genetic base of the disease. Some data were published on the detection of the locuses associated with the moyamoya disease on 3, 6, 8 and 17 chromosomes. In 2008 some data were published about the autosomal dominant inheritance (the gene was mapped to chromosome 17q25) of the moyamoya disease [119; 130; 197; 199; 225]. However, in the literature there is a description of the moyamoya disease in one of two twins, and this permitted the authors to conclude that this disease was not rigidly determined [256]. In one of their latest works J. Ma et al. report (China, 2013) that while studying the associative genetic predisposition, they have found the connection between the moyamoya disease and genic polymorphisms in RNF213 gene (p.R4859K and p.R4810K), which is more common in Japan and Korea, but less common in China [171].
Despite these studies, the genetic screening in the moyamoya disease has not become widely common, because its efficacy was not proved [233]. As of today, the researchers studying this problem think that this disease has a multi-factor nature [266].
As already stated above, the pathophysiology of the moyamoya disease consists in the gradual constriction of major stems of basilar intracranial arteries due to deposits of lipids in the intima in the absence of inflammation signs. The middle layer of arteries is thinned, the adventitia is not involved into the process. Similar changes in vessels may be observed in other organs, which shows that the vascular impairment has a systemic nature. The involvement of the immune system into the process is not ruled out [270]. In some authors’ opinion, inflammatory proteins participate in the development of the disease [287]. Anyway, intra- and extra-cranial vascular anastomoses are formed on the base of the brain during the progressive occlusion process in the vessels of the Willis’ circle [270; 277], which, to a certain degree, compensates for critical decrease of the regional cerebral blood flow, but leads to a gradual growth of chronic cerebral ischemia mostly in the cortical sections of big hemispheres [208; 266; 291]. The anastomotic capillary network is disappearing, while collaterals are developing from the ECA (meningeal collaterals are called «a magic network») [208; 291]. Arterial aneurysms frequently occur in the moyamoya disease. According to some data, the detection rate of VBS aneurysms reaches 62%, which several times exceeds the incidence of this pathology in the population (5—15%) [196; 270].
The unique character of clinical manifestations of the moyamoya disease and syndrome consists in the fact that this pathology may reveal both as an ischemic CVD and intracranial hemorrhages. Also, both these variants may occur in the same patient during his life [192; 196; 270]. In 1990 Y. Matsushima offered the moyamoya disease classification based on its clinical course:
Type 1: revealing as rare TIAs – 2 times per month or more seldom;
Type 2: revealing as frequent TIAs – 2 times per month or more often;
Type 3: revealing as a minor stroke (with regressive neurologic deficiency within 2—3 weeks). Small ischemic lesions may be found on a brain CT;
Type 4: revealing as a progressive stroke (gradual growth of neurologic deficiency with time);
Type 5: revealing as a complete stroke resulting in the formation of a persistent neurologic deficiency; extensive ischemic lesions are found on the cerebral tissue during the brain CT and MRI.
Types 1—5 are referred to ischemic variant of the disease course.
Type 6: the disease reveals as a hemorrhagic CVD due to a rupture of anastomotic network vessels [178].
C. Mohanty et al. (India, 2013) report 11 incidents of an unusual course of the moyamoya disease, when both hemorrhagic and ischemic CVD lesions are noted in the same hemisphere of a patient either simultaneously or at different periods of time [186].
According to foreign data, the moyamoya disease mortality is higher in adults than in children (10 and 4.3% respectively). Hemorrhages were the cause of death in 56% of 9 perished children. With the surgical treatment a favorable prognosis is noted in 58% of cases [268].
4. Clinical manifestations of cerebrovascular diseases in children
Clinical manifestations of acute cerebrovascular diseases in the carotid system of children are typical enough, and they reveal in a focal neurologic deficiency with developing motor disturbances (87—95%), disturbances of speech, sensibility and vision as well as other symptoms corresponding to the location of cerebral tissue lesions. In massive strokes during the acute period of the disease, as a rule, general neurological symptoms are more marked, which is caused by edema and dislocation of the brain. When the ischemic zone is small, the focal symptoms develop in the background of the generally normal state. The clinical picture of a cerebrovascular disease in a child may be atypical: nausea, vomit and depression of consciousness may be replaced with agitation or body temperature rise and convulsions (19—58%).
For obtaining more objective data on the severity of clinical manifestations of the ACVD and assessing the neurologic deficiency changes in acute and rehabilitation periods of the stroke, the PedNIHSS (Pediatric National Institute of Health Stroke Scale) scale is used, which has showed its consistence with the NIHSS scale [175; 235]. During the period of residual effects, American researchers apply the PSOM scale (Pediatric Stroke Outcome Measure) [37; 107]. These scales are rather extensive, but easy to fill in, so they can be automated and introduced into the patient examination standard. The obstacle for their implementation in the RF territory is the need for training of specialists in the estimation methodology proper as well as the need for validation of evaluation and measurement scales.
A paroxysmal syndrome often becomes the first symptom of any type of a CVD in children. More frequent registration of the paroxysmal syndrome is noted in the infant group [60; 69; 81; 291]. In children, the paroxysmal syndrome is typical not only for a stroke, but also for various brain damages (mass lesions, etc.). Due to its high diagnostic significance, it is recommended by the American Epilepsy Society as a mandatory indication for brain MRI [122].
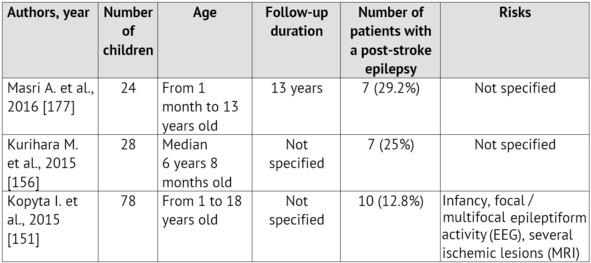
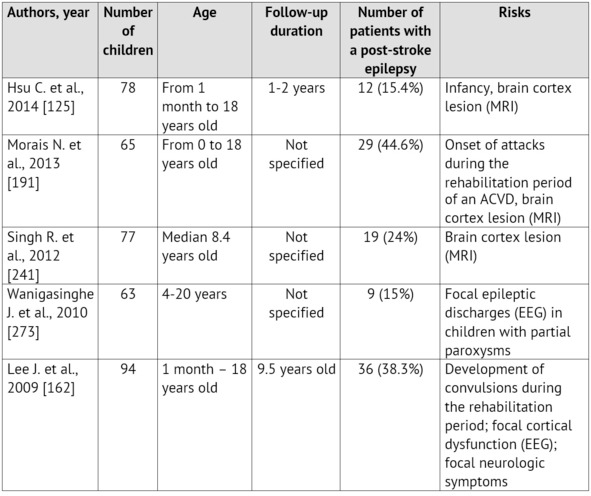
The emergence of convulsions at the onset of a cerebrovascular pathology is a relatively unfavorable sign. It is proved that it is specifically the ACVD manifestation in paroxysms recurring soon, that is associated with the unfavorable prognosis regarding the patient rehabilitation and the restoration of the focal neurologic deficiency as well as with the risk of emergence and severe course of symptomatic epilepsy [99; 125; 259]. Normally, convulsions are not the only manifestation of an ACVD. A focal neurologic deficiency develops either alongside with them, or in the later periods [35; 220; 280]. The largest samplings of patients with an ACVD and formation of a post-stroke epilepsy during the subsequent period of the disease are presented in Table 1.
Table 1: Epilepsy formation risk in children, who had suffered an ischemic stroke (according to literature data from 2010 till 2015)
On the other hand, the first manifestations of the disease may be rather non-specific: an isolated degradation of consciousness level or a headache, which, considering age, difficulties in awareness and in verbalization of unusual symptoms by the child itself as well as lack of «stroke» alertness in pediatric neurologists, leads to an essential delay in neuroimaging and ACVD diagnosing [24; 60; 63; 67; 179]. These circumstances often lead to a rather paradoxical situation called «clinicodiagnostic scissors»: children with CVD symptoms are hospitalized promptly enough, but they do not get adequate verification of the diagnosis, including an instrumental one, and, therefore, they do not get the treatment.
Pediatric patients find themselves in a hospital, on the average, within the first three hours from the onset of the ACVD symptoms, but they get to the neuroradiologists within 8 hours, whereas for adult patients these deadlines are 8 and 2 hours respectively [63; 103; 179; 247]. Transient motor and/or sensorial disturbances in the structure of partial attacks lead to the initial diagnoses, which are most common in children (epilepsy, neuroinfection, crainio-cerebral injury, etc.) and which, therefore, occupy top positions in the immediate memory of emergency phase doctors [103; 247].
During the analysis of the clinical picture in children with IS, examined by specialists of the FSBEI of Higher Professional Education «Urals State Medical University», the following data on the varying occurrence of clinical symptoms were obtained in 162 children (Table 4). Within the first 24 hours of the disease the comparability was noted between the registration rates of general cerebral and focal neurological symptoms. During the acute period of the IS, the most typical combination of symptoms in children were the degradation of consciousness level and the central pareses of limbs and mimic muscles.
Practically, every fourth child at an age enabling the adequate assessment of these symptoms had signs of ataxia and speech disturbances. Thus, the most prominent combinations of symptoms, which form the diagnostic rules such as «Give me five»1 and FAST2 in stroke diagnostics at the age typical for IS and TIA, can also be successfully applied in pediatric practice.
The spectrum of focal neurologic symptoms in patients was consistent with the blood supply systems and the infarction location. In the hospital stay period, the neurologic deficiency persistence had a direct positive connection with the size of the stroke zone on CT or MRI (r=0.56, p <0.05).
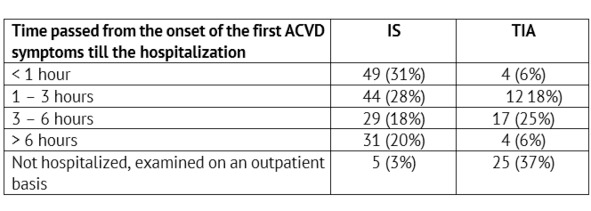
The rate of admitting children with verified IS (n=158) and TIA (n=62) to the specialized care delivery stage was analyzed (Table 2).
Thus, only a little more than half of the children with IS get to the specialized care delivery stage within the reported deadlines accepted for hospitalization of adults – within the boundaries of the so called «time slot» (up to three hours) – 59% (n=93), when thrombolysis is possible. For children with TIA the onset of focal or general cerebral symptoms did not remain unnoticed also in more than a half of cases – 60% (n=37). On the other hand, a short-term and transient nature of symptoms in TIA have led to the lack of the emergency hospitalization and to scheduled health-seeking in 40% of children in this group (n=25).
Blood circulation diseases of the VBS have such typical symptoms as vertigo with nausea and vomit, disorder of static equilibrium and gait, ataxia in limbs and nystagmus, and such less typical symptoms as pareses in the limbs and various sensibility variations, lesions of cerebral nerves (CN) caused by a cerebrovascular disease in the brain stem. In ischemia of occipital lobes there may be some disturbances of visual functions [17].
Table 2: Time passed from the onset of the first ACVD symptoms till the admission to an emergency / neurology specialized healthcare facility
A chronic cerebrovascular ischemia most often manifests itself in signs of a dyscirculatory encephalopathy (DEP) characterized by diffuse-type headaches, vertigo, tinnitus, memory derangements, emotional lability, increased fatigability and performance impairment, sleep disorder. In DEP, general symptoms prevail without focal neurologic symptoms: severe headaches (77%), increased fatigability (68%), hypomnesia (44%) [17]. Decreased learning capacity is typical for such children.
Regarding the specific features of the clinical course of some types of the pathology, it should be mentioned that clinical manifestations of stenosis and occlusion of major cerebral vessels do not have any specific features and can manifest themselves as various ischemic cerebrovascular diseases. A moderate decrease of the cerebral blood flow usually does not manifest itself clinically (asymptomatic disease course), or it may be accompanied by some unspecific complaints.
At the onset of the moyamoya disease and syndrome, their clinical manifestations are rather diverse, and they may resemble the clinical manifestations of cerebrovascular disturbances in pathologic deformations of major cerebral vessels, thrombosis and atherosclerosis of intracranial arteries as well as the manifestations of other diseases (epilepsy, malformations of cerebral vessels, subarachnoid and intra-cerebral hemorrhages of various genesis [192; 196; 270].
Headache is mentioned as a manifesting symptom in a variety of studies on the moyamoya disease occurring in children too. In literature, there is even an individual notion – a headache associated with the moyamoya disease (HAMD) [141; 233; 238; 288]. Headache is often the only symptom at the onset of this disease. The headache is supposed to be caused by a compensatory dilatation of meningeal and leptomeningeal arteries, which can stimulate nociceptive receptors of dura mater of brain (DMB). The headache may have a migraine-like nature and be resistant to a drug therapy. However, this symptom usually is not considered as a fatal sign. In most patients, the headache regresses after the surgery [71; 136; 238].
The subsequent joining of transient focal neurologic symptoms is often considered by neurologists as a manifestation of sub- or de-compensation of residual organic background under the effect of school loads, intense sports activities, viral infections, vaccinations, etc. They are acknowledged as TIAs most often retrospectively, after the verification of the moyamoya disease. A short-term and transient nature of symptoms in children, often combined with the inability to describe their «unusual» complaints verbally, result in delayed help-seeking and late hospitalization. According to literature data, the delayed diagnosing is noted in all patients, and it may exceed two years. The moyamoya disease is usually identified only after the child has suffered a typical IS, which is followed by various neuroimaging examinations. Clinical manifestations in children with the moyamoya disease are distributed in the occurrence rate as follows: ischemic symptoms – 80% of cases (including strokes – 40% and transitory ischemic attacks – 41%) [21; 80; 224; 242; 254]; epilepsy – 5%, intracranial hemorrhages – 2.5%; other symptoms – 12.5% of cases (headache, motor disturbances, or combined symptoms) [72; 80; 224; 242; 254].
5. Diagnostics of pediatric stroke
A nation-wide Russian recommended list of diagnostic procedures aimed at differential diagnostics of a CVD at an early age does not exist. It is actively discussed and formed in some individual centers. The variability of the CVD causes considerably hinders the diagnostic search. During the most acute and acute disease periods, the efforts are focused on identifying the pathogenic variant of the CVD, primarily, on identification of the most frequent diseases, whose therapy can be started immediately (cardiac pathology, congenital clotting disorders, congenital and acquired pathology of cerebral vessels) with due regard for the age [22; 34]. If the cause has not been determined, it is recommended afterwards to rule out consecutively other, less common, causes of the CVD at an early age [7; 9; 22; 24; 134].
It is known that, despite a rather well-defined organization of the diagnostic process in foreign clinics, about 20% of ischemic strokes remain etiologically unexplained. The similar national indicator reaches 65 – 70% [161; 174; 280]. Despite the high cost of a laboratory-based instrumental examination and its long duration, such an examination must be held. The earliest clarification of the cause of an ACVD in a child is considered a high-priority and the most important mission of a diagnostic search at any phase of the disease. The accurate clarification of the etiology of an ischemic ACVD determines the focus of the corrective drug therapy, the system of preventive measures and the prognosis regarding the further course and recurrence of the disease.
Based on the FSBEI of Higher Professional Education «Urals State Medical University», a list of diagnostic activities, which are due to be held for examination of children with IS and TIA at an emergency (inpatient) stage, was developed and implemented into a routine practice (Table 3).
It must be noted that a consecutive clinical, laboratory-based and instrumental examination with the fulfillment of all items proposed above should be held even in the cases, when the cause of a stroke or TIA seems evident. This is due to the fact that, beside an evident cause, the presence of other, equally important, risks may be possible, which could lead to a pathology of the blood coagulation system. It is just these individual risk combinations, which, under the effect of the initiating agents, facilitate the realization of genetically determined prothrombotic readiness and lead to the formation of a focal spot of an infarction or TIA in children.

