 полная версия
полная версияThe Elements of Agriculture
Gluten absorbs large quantities of water, which causes it to swell to a great size, and become full of holes. Flour which contains much gluten, makes light, porous bread, and is preferred by bakers, because it absorbs so large an amount of water.
What is the result if a field be deficient in nitrogen?
The protein substances are necessary to animal and vegetable life, and none of our cultivated plants will attain maturity (complete their growth), unless allowed the materials required for forming this constituent. To furnish this condition is the object of nitrogen given to plants as manure. If no nitrogen is supplied the protein substances cannot be formed, and the plant must cease to grow.
When on the contrary ammonia is given to the soil (by rains or otherwise), it furnishes nitrogen, while the carbonic acid and water yield the other constituents of protein, and a healthy growth continues, provided that the soil contains the mineral matters required in the formation of the ash, in a condition to be useful.
The wisdom of this provision is evident when we recollect that the protein substances are necessary to the formation of muscle in animals, for if plants were allowed to complete their growth without a supply of this ingredient, our grain and hay might not be sufficiently well supplied with it to keep our oxen and horses in working condition, while under the existing law plants must be of nearly a uniform quality (in this respect), and if a field is short of nitrogen, its crop will not be large, and of a very poor quality, but the soil will produce good plants as long as the nitrogen lasts, and then the growth must cease.9
ANIMALSThat this principle may be clearly understood, it may be well to explain more fully the application of the proximate constituents of plants in feeding animals.
Of what are the bodies of animals composed?
What is the office of vegetation?
What part of the animal is formed from the first class of proximates?
From the second?
Which contains the largest portions of inorganic matter, plants or animals?
Must animals have a variety of food, and why?
Animals are composed (like plants) of organic and inorganic matter, and every thing necessary to build them up exists in plants. It seems to be the office of the vegetable world to prepare the gases in the atmosphere, and the minerals in the earth for the uses of animal life, and to effect this plants put these gases and minerals together in the form of the various proximates (or compound substances) which we have just described.
In animals the compounds containing no nitrogen comprise the fatty substances, parts of the blood, etc., while the protein compound, or those which do contain nitrogen, form the muscle, a part of the bones, the hair, and other portions of the animal.
Animals contain a larger proportion of inorganic matter than plants do. Bones contain a large quantity of phosphate of lime, and we find other inorganic materials performing important offices in the system.
In order that animals may be perfectly developed, they must of course receive as food all of the materials required to form their bodies. They cannot live if fed entirely on one ingredient. Thus, if starch alone be eaten by the animal, he might become fat, but his strength would soon fail, because his food contains nothing to keep up the vigor of his muscles. If on the contrary the food of an animal consisted entirely of gluten, he might be very strong from a superior development of muscle, but would not be fat. Hence we see that in order to keep up the proper proportion of both fat and muscle in our animals (or in ourselves), the food must be such as contains a proper proportion of the two kinds of proximates.
Why is grain good for food?
On what does the value of flour depend?
Is there any relation between the ashy part of plants and those of animals?
How may we account for unhealthy bones and teeth?
It is for this reason that grain, such as wheat for instance, is so good for food. It contains both classes of proximates, and furnishes material for the formation of both fat and muscle. The value of flour depends very much on the manner in which it is manufactured. This will be soon explained.
What is a probable cause of consumption?
What is an important use of the first class of proximates?
What may lungs be called?
Explain the production of heat during decomposition.
Why is the heat produced by decay not perceptible?
Apart from the relations between the proximate principles of plants, and those of animals, there exists an important relation between their ashy or inorganic parts; and, food in order to satisfy the demands of animal life, must contain the mineral matter required for the purposes of that life. Take bones for instance. If phosphate of lime is not always supplied in sufficient quantities by food, animals are prevented from the formation of healthy bones. This is particularly to be noticed in teeth. Where food is deficient of phosphate of lime, we see poor teeth as a result. Some physicians have supposed that one of the causes of consumption is the deficiency of phosphate of lime in food.
Why is the heat produced by combustion apparent?
Explain the production of heat in the lungs of animals?
Why does exercise augment the animal heat?
Under what circumstances is the animal's own fat used in the production of heat?
The first class of proximates (starch, sugar, gum, etc.), perform an important office in the animal economy aside from their use in making fat. They constitute the fuel which supplies the animal's fire, and gives him his heat. The lungs of men and other animals may be called delicate stoves, which supply the whole body with heat. But let us explain this matter more fully. If wood, starch, gum, or sugar, be burned in a stove, they produce heat. These substances consist, as will be recollected, of carbon, hydrogen, and oxygen, and when they are destroyed in any way (provided they be exposed to the atmosphere), the hydrogen and oxygen unite and form water, and the carbon unites with the oxygen of the air and forms carbonic acid, as was explained in a preceding chapter. This process is always accompanied by the liberation of heat, and the intensity of this heat depends on the time occupied in its production. In the case of decay, the chemical changes take place so slowly that the heat, being conducted away as soon as formed, is not perceptible to our senses. In combustion (or burning) the same changes take place with much greater rapidity, and the same amount of heat being concentrated, or brought out in a far shorter time, it becomes intense, and therefore apparent. In the lungs of animals the same law holds true. The blood contains matters belonging to this carbonaceous class, and they undergo in the lungs the changes which have been described under the head of combustion and decay. Their hydrogen and oxygen unite, and form the moisture of the breath, while their carbon is combined with the oxygen of the air drawn into the lungs, and is thrown out as carbonic acid. The same consequence—heat—results in this, as in the other cases, and this heat is produced with sufficient rapidity for the animal necessities. When an animal exercises violently, his blood circulates with increased rapidity, thus carrying carbon more rapidly to the lungs. The breath also becomes quicker, thus supplying increased quantities of oxygen. In this way the decomposition becomes more rapid, and the animal is heated in proportion.
Thus we see that food has another function besides that of forming animal matter, namely to supply heat. When the food does not contain a sufficient quantity of starch, sugar, etc., to answer the demands of the system the animal's own fat is carried to the lungs, and there used in the production of heat. This important fact will be referred to again.
CHAPTER VII
LOCATION OF THE PROXIMATES AND VARIATIONS IN THE ASHES OF PLANTSOf what proximate are plants chiefly composed?
What is the principal constituent of the potato root?
Of the carrot and turnip?
What part of the plant contains usually the most nutriment?
Let us now examine plants with a view to learning the location of the various plants.
The stem or trunk of the plant or tree consists almost entirely of woody fibre; this also forms a large portion of the other parts except the seeds, and, in some instances, the roots. The roots of the potato contain large quantities of starch. Other roots such as the carrot and turnip contain pectic acid,10 a nutritious substance resembling starch.
It is in the seed however that the more nutritive portions of most plants exist, and here they maintain certain relative positions which it is well to understand, and which can be best explained by reference to the following figures, as described by Prof. Johnston:—
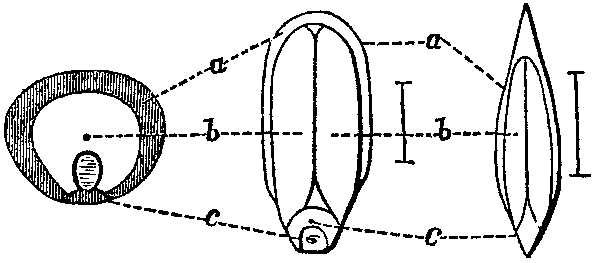
Fig. 1.
"Thus a shows the position of the oil in the outer part of the seed—it exists in minute drops, inclosed in six-sided cells, which consists chiefly of gluten; b, the position and comparative quantity of the starch, which in the heart of the seed is mixed with only a small proportion of gluten; c, the germ or chit which contains much gluten."11
Is the composition of the inorganic matter of different parts of the plant the same, or different?
What is the difference between the ash of the straw and that of the grain of wheat?
The location of the inorganic part of plants is one of much interest, and shows the adaptation of each part to its particular use. Take a wheat plant, for instance—the stalk, the leaf, and the grain, show in their ashes, important difference of composition. The stalk or straw contains three or four times as large a proportion of ash as the grain, and a no less remarkable difference of composition may be noticed in the ashes of the two parts. In that of the straw, we find a large proportion of silica and scarcely any phosphoric acid, while in that of the grain there is scarcely a trace of silica, although phosphoric acid constitutes more than one half of the entire weight. The leaves contain a considerable quantity of lime.
What is the reason for this difference?
In what part of the grain does phosphoric acid exist most largely?
This may at first seem an unimportant matter, but on examination we shall see the use of it. The straw is intended to support the grain and leaves, and to convey the sap from the roots to the upper portions of the plant. To perform these offices, strength is required, and this is given by the silica, and the woody fibre which forms so large a proportion of the stalk. The silica is combined with an alkali, and constitutes the glassy coating of the straw. While the plant is young, this coating is hardly apparent, but as it grows older, as the grain becomes heavier, (verging towards ripeness), the silicious coating of the stalk assumes a more prominent character, and gives to the straw sufficient strength to support the golden head. The straw is not the most important part of the plant as food, and therefore requires but little phosphoric acid.
Why is Graham flour more wholesome than fine flour?
Are the ashes of all plants the same in their composition?
The grain, on the contrary, is especially intended as food, and therefore must contain a large proportion of phosphoric acid—this being, as we have already learned, necessary to the formation of bone—while, as it has no necessity for strength, and as silica is not needed by animals, this ingredient exists in the grain only in a very small proportion. It may be well to observe that the phosphoric acid of grain exists most largely in the hard portions near the shell, or bran. This is one of the reasons why Graham flour is more wholesome than fine flour. It contains all of the nutritive materials which render the grain valuable as food, while flour which is very finely bolted12 contains only a small part of the outer portions of the grain (where the phosphoric acid, protein and fatty matters exist most largely). The starchy matter in the interior of the grain, which is the least capable of giving strength to the animal, is carefully separated, and used as food for man, while the better portions, not being ground so finely, are rejected. This one thing alone may be sufficient to account for the fact, that the lives of men have become shorter and less blessed with health and strength, than they were in the good old days when a stone mortar and a coarse sieve made a respectable flour mill.
Another important fact concerning the ashes of plants is the difference of their composition in different plants. Thus, the most prominent ingredient in the ash of the potato is potash; of wheat and other grains, phosphoric acid; of meadow hay, silica; of clover, lime; of beans, potash, etc. In grain, potash (or soda), etc., are among the important ingredients.
Of what advantage are these differences to the farmer?
Of what are plants composed?
These differences are of great importance to the practical farmer, as by understanding what kind of plants use the most of one ingredient, and what kind requires another in large proportion, he can regulate his crops so as to prevent his soil from being exhausted more in one ingredient than in the others, and can also manure his land with reference to the crop which he intends to grow. The tables of analyses in the fifth section will point out these differences accurately.
CHAPTER VIII
RECAPITULATIONWe have now learned as much about the plant as is required for our immediate uses, and we will carefully reconsider the various points with a view to fixing them permanently in the mind.
Plants are composed of organic and inorganic matter.
What is organic matter? Inorganic?
Of what does organic matter consist? Inorganic?
How do plants obtain their organic food?
How their inorganic?
How is ammonia supplied? Carbonic acid?
Organic matter is that which burns away in the fire. Inorganic matter is the ash left after burning.
The organic matter of plants consists of three gases, oxygen, hydrogen and nitrogen, and one solid substance carbon (or charcoal). The inorganic matter of plants consists of potash, soda, lime, magnesia, sulphuric acid, phosphoric acid, chlorine, silica, oxide of iron, and oxide of manganese.
Plants obtain their organic food as follows:—Oxygen and hydrogen from water, nitrogen from some compound containing nitrogen (chiefly from ammonia), and carbon from the atmosphere where it exists as carbonic acid—a gas.
They obtain their inorganic food from the soil.
The water which supplies oxygen and hydrogen to plants is readily obtained without the assistance of manures.
Ammonia is obtained from the atmosphere, by being absorbed by rain and carried into the soil, and it enters plants through their roots. It may be artificially supplied in the form of animal manure with profit.
Carbonic acid is absorbed from the atmosphere by leaves, and decomposed in the green parts of plants under the influence of daylight; the carbon is retained, and the oxygen is returned to the atmosphere.
When plants are destroyed by combustion or decay, what becomes of their constituents?
How does the inorganic matter enter the plant?
Are the alkalies soluble in their pure forms?
Which one of them is injurious when too largely present?
How may sulphuric acid be supplied?
Is phosphoric acid important?
How must silica be treated?
From what source may we obtain chlorine?
When plants are destroyed by decay, or burning, their organic constituents pass away as water, ammonia, carbonic acid, etc., ready again to be taken up by other plants.
The inorganic matters in the soil can enter the plant only when dissolved in water. Potash, soda, lime, and magnesia, are soluble in their pure forms. Magnesia is injurious when present in too large quantities.
Sulphuric acid is often necessary as a manure, and is usually most available in the form of sulphate of lime or plaster. It is also valuable in its pure form to prevent the escape of ammonia from composts.
Phosphoric acid is highly important, from its frequent deficiency in worn-out soils. It is available only under certain conditions which will be described in the section on manures.
Silica is the base of common sand, and must be united to an alkali before it can be used by the plant, because it is insoluble except when so united.
Chlorine is a constituent of common salt (chloride of sodium), and from this source may be obtained in sufficient quantities for manurial purposes.
What is the difference between peroxide and protoxide of iron?
How must the food of plants be supplied?
What takes place after it enters the plant?
What name is given to the compounds thus formed?
How are proximates divided?
Which class constitutes the largest part of the plant?
Of what are animals composed, and how do they obtain the materials from which to form their growth?
Oxide of iron is iron rust. There are two oxides of iron, the peroxide (red) and the protoxide (black). The former is a fertilizer, and the latter poisons plants.
Oxide of manganese is often absent from the ashes of our cultivated plants.
The food of plants, both organic and inorganic, must be supplied in certain proportions, and at the time when it is required. In the plant, this food undergoes such chemical changes as are necessary to growth.
The compounds formed by these chemical combinations are called proximates.
Proximates are of two classes, those not containing nitrogen, and those which do contain it.
The first class constitute nearly the whole plant.
The second class, although small in quantity, are of the greatest importance to the farmer, as from them all animal muscle is made.
Animals, like plants, are composed of both organic and inorganic matter, and their bodies are obtained directly or indirectly from plants.
What parts of the animal belong to the first class of proximates?
What to the second?
What is necessary to the perfect development of animals?
Why are seeds valuable for working animals?
What other important use, in animal economy, have proximates of the first class?
Under what circumstances is animal fat decomposed?
The first class of proximates in animals comprise the fat, and like tissues.
The second class form the muscle, hair, gelatine of the bones, etc.
In order that they may be perfectly developed, animals must eat both classes of proximates, and in the proportions required by their natures.
They require the phosphate of lime and other inorganic food which exist in plants.
Seeds are the best adapted to the uses of working animals, because they are rich in all kinds of food required.
Aside from their use in the formation of fat, proximates of the first class are employed in the lungs, as fuel to keep up animal heat, which is produced (as in fire and decay) by the decomposition of these substances.
When the food is insufficient for the purposes of heat, the animal's own fat is decomposed, and carried to the lungs as fuel.
The stems, roots, branches, etc., of most plants consist principally of woody fibre.
Their seeds, and sometimes their roots, contain considerable quantities of starch.
Name the parts of the plant in which the different proximates exist.
State what you know about flour.
Do we know that different plants have ashes of different composition?
The protein and the oils of most plants exist most largely in the seeds.
The location of the proximates, as well as of the inorganic parts of the plant, show a remarkable reference to the purposes of growth, and to the wants of the animal world, as is noticed in the difference between the construction of the straw and that of the kernel of wheat.
The reason why the fine flour now made is not so healthfully nutritious as that which contained more of the coarse portions, is that it is robbed of a large proportion of protein and phosphate of lime, while it contains an undue amount of starch, which is available only to form fat, and to supply fuel to the lungs.
Different plants have ashes of different composition. Thus—one may take from the soil large quantities of potash, another of phosphoric acid, and another of lime.
By understanding these differences, we shall be able so to regulate our rotations, that the soil may not be called on to supply more of one ingredient than of another, and thus it may be kept in balance.
How are farmers to be benefited by such knowledge?
The facts contained in this chapter are the alphabet of agriculture, and the learner should not only become perfectly familiar with them, but should also clearly understand the reasons why they are true, before proceeding further.
SECTION SECOND.
THE SOIL
CHAPTER I
What is a necessary condition of growth?
In the foregoing section, we have studied the character of plants and the laws which govern their growth. We learned that one necessary condition for growth is a fertile soil, and therefore we will examine the nature of different soils, in order that we may understand the relations between them and plants.
What is a fixed character of soils?
How is the chemical character of the soil to be ascertained?
What do we first learn in analyzing a soil?
How do the proportions of organic or inorganic parts of soils compare with those of plants?
Of what does the organic part of soils consist?
The soil is not to be regarded as a mysterious mass of dirt, whereon crops are produced by a mysterious process. Well ascertained scientific knowledge has proved beyond question that all soils, whether in America or Asia, whether in Maine or California, have certain fixed properties, which render them fertile or barren, and the science of agriculture is able to point out these characteristics in all cases, so that we can ascertain from a scientific investigation what would be the chances for success in cultivating any soil which we examine.
The soil is a great chemical compound, and its chemical character is ascertained (as in the case of plants) by analyzing it, or taking it apart.
We first learn that fertile soils contain both organic and inorganic matter; but, unlike the plant, they usually possess much more of the latter than of the former.
In the plant, the organic matter constitutes the most considerable portion of the whole. In the soil, on the contrary, it usually exists in very small quantities, while the inorganic portions constitute nearly the whole bulk.
Can the required proportion be definitely indicated?
From what source is the inorganic part of soils derived?
Do all soils decompose with equal facility?
How does frost affect rocks?
Does it affect soils in the same way?
The organic part of soils consists of the same materials that constitute the organic part of the plants, and it is in reality decayed vegetable and animal matter. It is not necessary that this organic part of the soil should form any particular proportion of the whole, and indeed we find it varying from one and a half to fifty, and sometimes, in peaty soils, to over seventy per cent. All fertile soils contain some organic matter, although it seems to make but little difference in fertility, whether it be ten or fifty per cent.
The inorganic part of soils is derived from the crumbling of rocks. Some rocks (such as the slates in Central New York) decompose, and crumble rapidly on being exposed to the weather; while granite, marble, and other rocks will last for a long time without perceptible change. The causes of this crumbling are various, and are not unimportant to the agriculturist; as by the same processes by which his soil was formed, he can increase its depth, or otherwise improve it. This being the case, we will in a few words explain some of the principal pulverizing agents.


