
Полная версия
All sciences. №7, 2023. International Scientific Journal

Fig. 1. Cadmium telluride crystal
The use of this element is really popular at the moment when creating solar panels, ionizing radiation detectors and photodetectors, but the mathematical basis of these phenomena still requires consideration. This material, in its normal state, is solid with a molar mass of 240.01 g / mol and a density of 5.85 g / cm3, has a melting point of 1092 degrees Celsius after its formation with a cubic structure or a sphalerite structure, also popularly popular as zinc blende.
In the formed material, the coefficient of linear thermal expansion is 5,9*10—6 1/ K when the temperature value reaches 293 K. The Young’s modulus of such a material reaches 52 Gpa with a Poisson’s ratio of 0.41. Another, for some cases, favorable moments is the circumstance of its transparency for infrared radiation from 830 nm, but negative if it is necessary to detect such classes of radiation. It should be noted that this radiation depends on an energy close to the band gap of the material of 1.5 eV at 300 K, which causes its transparency for this kind of radiation corresponding to 20 microns.
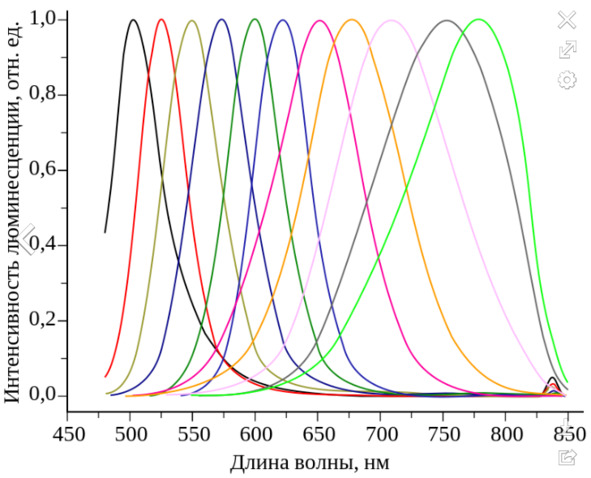
Fig. 2. Shift of fluorescence spectra in cadmium telluride
This element also has the property of fluorescence, but reaches its peak only at 790 nm. This law is effective only for massive crystals, but when their size decreases comparatively and can reach the state of reduction to quantum dots, the peak of fluorescence begins to shift by a certain value, being already in the ultraviolet range. Most of all, this dependence is personified by the fluorescence spectrum of cadmium telluride for various sizes, where the size of colloidal particles increases from about 2 to 20 nm, and some quantum well appears in the face of the reason for this peak shift (Fig. 2).
Among the chemical properties of this compound, it is not worth saying quite a lot and it is quite enough to note that it is bad it dissolves in water, has the property of interacting even with weak acids with the release of hydrogen telluride and the formation of the corresponding salt, which is quite obvious.
Based on all the presented physico-chemical descriptions of this compound, as well as finding compliance with the physico-mathematical laws of photovoltaic phenomena, it is possible in a comparative analysis to talk about the very favorable suitability of this material for the role of a semiconductor photovoltaic base for such devices with relatively high efficiency. But it is worth saying that further improvement of this technology is inevitable and requires more detailed further consideration.
Used literature
1. Bovin L. A and others . Physics of compounds a-2 b-6 / edited by A. N. Georgobiani, M. K. Sheinkman. – M.: Nauka, Gl. ed. Phys.-mat. Lit., 1986. – 319 p.
2. Anselm, A. I. Introduction to the theory of semiconductors / A. I. Anselm. – L.: Nauka, 1978. – 616 p.
3. Anselm, A. I. Introduction to the theory of semiconductors / A. I. Anselm. – M.: Lan, 2008. – 624 p.
4. Anselm, A. I. Introduction to the theory of semiconductors / A. I. Anselm. – Moscow: Ogni, 1978. – 770 p.
5. Atia, M. Geometry and physics of knots / M. Atia. – Moscow: SPb. [et al.]: Peter, 1995. – 963 p.
6. Borisov, E. The key to the sun. Stories about semiconductors / E. Borisov, I. Pyatnova. – L.: Molodaya Gvardiya, 1997. – 304 p.
7. Dunlap, U. Introduction to semiconductor physics / U. Dunlap. – M.: Publishing House of Foreign Literature, 2011. – 430 p.
8. Zeldovich, Ya. B. Higher mathematics for beginners and its applications to physics / Ya. B. Zeldovich. – Moscow: RSUH, 1983. – 794 p.
9. Zeldovich, Ya. B. Higher mathematics for beginning physicists and technicians / Ya. B. Zeldovich, I. M. Yaglom. – Moscow: IL, 1982. – 108 p.
10. Ioffe, A. F. Selected works (volume 2). Radiation, electrons, semiconductors: monogr. / A. F. Ioffe. – Moscow: Nauka, 1976. – 552 p.
11. Kurchatov, I. V. I. V. Kurchatov. Collection of scientific papers in 6 volumes. Volume 1. Early works. Dielectrics. Semiconductors / I. V. Kurchatov. – L.: Nauka, 2005. – 576 p.
12. Ladyzhenskaya, O. A. Boundary value problems of mathematical physics / O. A. Ladyzhenskaya. – Moscow: Gostekhizdat, 1975. – 810 p.
13. Levinstein, M. E. Acquaintance with semiconductors / M. E. Levinstein, G. S. Simin. – M.: The main editorial office of physical and mathematical literature of the publishing house "Nauka", 1984. – 240 p.
14. Levinstein, M. E. Acquaintance with semiconductors / M. E. Levinstein, G. S. Simin. – M.: Institute of Computer Research, 2004. – 208 p.
15. Mikhlin, S. G. Course of mathematical physics / S. G. Mikhlin. – Moscow: Higher School, 2005. – 947 p.
16. New semiconductor devices: Chemistry, physics, semiconductor technology. – M.: Gostekhizdat, 1975. – 748 p.
17. Ormont, B. F. Introduction to physical chemistry and crystal chemistry of semiconductors / B. F. Ormont. – M.: Higher School, 1975. – 490 p.
18. Rectoris, K. Variational methods in mathematical physics and engineering / K. Rectoris. – Moscow: Higher School, 1985. – 363 p.
19. Slater, J. Dielectrics. Semiconductors. Metals / J. Slater. – M.: Mir, 2001. – 648 p.
20. Ugai, Ya. A. Introduction to semiconductor chemistry / Ya. A. Ugai. – M.: Higher School, 1975. – 302 p.
21. Frank, F. Differential and integral equations of mathematical physics (part 2) / F. Frank, R. Mises. – Moscow: IL, 1990. – 467 p.
THE USE OF ELECTROMAGNETIC FIELDS TO IMPROVE THE OVERALL EFFICIENCY OF PLANT GROWTH IN THE PHYSICO-BIOLOGICAL SENSE
UDC 581.132
Kadyrbergenov Fozil Kudratovich
2nd year student of the Department of "Electronics and Instrumentation" of the Faculty of Computer Design Systems of the Fergana Polytechnic Institute
Ferghana Polytechnic Institute, Ferghana, Uzbekistan
Annotation. The development of various kinds of achievements in modern science leads to an acceleration of the process of determining a new kind of invention and the impact of one of the phenomena on others. The proof of this can be a technology that has never been mentioned, but is only now actively developing as a separate method, namely, the technology of accelerating plant growth through the influence of electromagnetic fields on them and on the soil occupied by them.
Keywords: electromagnetic field, physico-biological processes, photosynthesis, growth acceleration, plants.
Аннотация. Развитие самого разного рода достижений в современной науке ведёт к ускорению процесса определения нового рода изобретений и воздействия одного из явлений на другие. Доказательством тому может служить технология, никогда упоминаемая, но лишь ныне активно развивающаяся в роли отдельного способа, а именно технология ускорения роста растений посредством влияния на них и на занимаемую ими почву электромагнитных полей.
Ключевые слова: электромагнитное поле, физико-биологические процессы, фотосинтез, ускорение роста, растения.
The process of plant growth itself, as is known, is based on a whole array of various physical, chemical and biological phenomena, each of which contributes to the overall growth, however, one of the processes can affect quite quickly, the other on the contrary – makes less effort, but also remains important. Responsible for their specific area in the whole overall process. The growth itself is explained by the active cell division of the plant itself, for which it expends certain energy resources, as the same ATP molecules, as well as biological ones. For example, for the initial synthesis of proteins, after contact with deoxyribonucleic acid (DNA) with transcriptase in the nucleolus and the formation of information ribonucleic acid (i-RNA), as well as its subsequent transfer through the cytoplasm to ribosomes, where the synthesis of all necessary proteins is carried out, it is necessary to introduce "materials" there as transport RNA.
In order for each of the cells to receive the necessary material in the role of food, which the cell absorbs through its shells and directs the necessary inhibitor proteins that break it down into parts, as well as in the Golgi complex, enzymes that form from them necessary for reproduction. Moreover, when a cell receives everything necessary for the continuation of the "genus", its nucleus first begins to divide, of course, with the copying of DNA and chromosomes in the nuclei, although the loss of transcriptase at the ends. Further, the necessary vacuoles and other parts of the cell also begin to be equally distributed, eventually increasing the cell to the state until finally the outer shell forms two new cells from this mass, so one cell divides and thus the whole plant grows, if necessary, receiving additional chemical and biological "signals" for division in one or another form. For example, turning already from stem cells into cells of leaves, petals, etc.
When the process itself is more or less presented, although not in great detail, it is worth turning to ways to increase the speed of this growth. In order to obtain nutrients, a large number of familiar noble plants use their roots, most often absorbing water rich in minerals and enzymes dissolved in it. And also, it receives energy from the Sun using photosynthesis, a complex process during which a plant absorbs carbon dioxide through its leaves and releases oxygen, although in its normal state it also absorbs oxygen, like most living organisms. When all the necessary enzymes are collected, chlorophyll comes into play – perfectly accepting solar radiation and converting it into ATP molecules, and those in turn help transform the simplest and elementary compounds received into full-fledged enzymes necessary for plant growth.
An increase in sunlight would lead to the fact that the plant could get burned, as this would lead to rapid evaporation of moisture on its surface and rapid drying. So, the plant, protecting its stem, grows leaves, also providing large areas for absorbing the gases it needs, including carbonate from the air. If we resort to the method of saturating the atmosphere surrounding the plant with various gases necessary for it, then their overdose would lead to the fact that some processes could accelerate or increase their volume for a short time, but others would simply stop, again leading to the death of the plant. For example, if you enrich the surrounding space with carbonate, in order for the plant to produce photosynthesis better, this would lead to the fact that it simply could no longer be in its usual state – absorbing oxygen and releasing this very carbon dioxide, carbonate, which would slow down most processes.
Конец ознакомительного фрагмента.
Текст предоставлен ООО «Литрес».
Прочитайте эту книгу целиком, купив полную легальную версию на Литрес.
Безопасно оплатить книгу можно банковской картой Visa, MasterCard, Maestro, со счета мобильного телефона, с платежного терминала, в салоне МТС или Связной, через PayPal, WebMoney, Яндекс.Деньги, QIWI Кошелек, бонусными картами или другим удобным Вам способом.

