 полная версия
полная версияThe Elements of Agriculture
If quick-lime is applied directly to the soil, it absorbs first moisture, and then carbonic acid, becoming finally a powdered carbonate of lime.
One ton of carbonate of lime contains 11¼ cwt. of lime; the remainder is carbonic acid. One ton of slaked lime contains about 15 cwt. of lime; the remainder is water.
Hence we see that lime should be burned, and not slaked, before being transported, as it would be unprofitable to transport the large quantity of carbonic acid and water contained in carbonate of lime and slaked lime. The quick-lime may be slaked, and carbonated after reaching its destination, either before or after being applied to the land.
What is the best form for immediate action on the inorganic matter in the soil?
For most other purposes?
As has been before stated, much is gained by slaking lime with salt water, thus imitating the lime and salt mixture. Indeed in many cases, it will be found profitable to use all lime in this way. Where a direct action on the inorganic matters contained in the soil is desired, it may be well to apply the lime directly in the form of quick-lime; but, where the decomposition of the vegetable and animal constituents of the soil is desired, the correction of sourness, or the supplying of lime to the crop, the mixture with salt would be advisable.
The amount of lime required by plants is, as was before observed, usually small compared with the whole amount contained in the soil; still it is not unimportant.
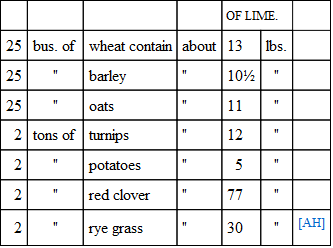
[AH] The straw producing the grain and the turnip and potato tops contain more lime than the grain and roots.
What is the best guide concerning the quantity of lime to be applied?
What is said of the sinking of lime in the soil?
What is plaster of Paris composed of?
Why is it called plaster of Paris?
The amount of lime required at each application, and the frequency of those applications, must depend on the chemical and mechanical condition of the soil. No exact rule can be given, but probably the custom of each district—regulated by long experience—is the best guide.
Lime sinks in the soil; and therefore, when used alone, should always be applied as a top dressing to be carried into the soil by rains. The tendency of lime to settle is so great that, when cutting drains, it may often be observed in a whitish streak on the top of the subsoil. After heavy doses of lime have been given to the soil, and have settled so as to have apparently ceased from their action, they may be brought up and mixed with the soil by deeper plowing.
Lime should never be mixed with animal manures, unless in compost with muck, or some other good absorbent, as it is liable to cause the escape of their ammonia.
PLASTER OF PARISPlaster of Paris or Gypsum (sulphate of lime) is composed of sulphuric acid and lime in combination. It is called 'plaster of Paris,' because it constitutes the rock underlying the city of Paris.
Is it a constituent of plants?
What else does it furnish them?
How does it affect manure?
How does it produce sorrel in the soil?
How may the acidity be overcome?
It is a constituent of many plants. It also furnishes them with sulphur—a constituent of the sulphuric acid which it contains.
It is an excellent absorbent of ammonia, and is very useful to sprinkle around stables, poultry houses, pig-styes, and privies, where it absorbs the escaping gases, saving them for the use of plants, and purifying the air, thus rendering stables, etc., more healthy than when not so supplied.
It has been observed that the extravagant use of plaster sometimes induces the growth of sorrel. This is probably the case only where the soil is deficient in lime. In such instances, the lime required by plants is obtained by the decomposition of the plaster. The lime enters into the construction of the plant, and the sulphuric acid remains free, rendering the soil sour, and therefore in condition to produce sorrel. In such a case, an application of lime will correct the acid by uniting with it and converting it into plaster.
CHLORIDE OF LIMEWhat does chloride of lime supply to plants?
How does it affect manures?
How may it be used?
How may magnesia be supplied, when wanting?
What care is necessary concerning the use of magnesia?
Chloride of lime is a compound of lime and chlorine. It furnishes both of these constituents to plants, and it is an excellent absorbent of ammonia and other gases arising from decomposition—hence its usefulness in destroying bad odors, and in preserving fertilizing matters for the use of crops.
It may be used like plaster, or in the decomposition of organic matters, where it not only hastens decay, but absorbs and retains the escaping gases. It will be recollected that chloride of lime is one of the products of the lime and salt mixture.
Lime in combination with phosphoric acid forms the valuable phosphate of lime, of which so large a portion of the ash of grain, and the bones of animals, is formed. This will be spoken of more at length under the head of 'phosphoric acid.'
MAGNESIAMagnesia is a constituent of vegetable ashes, and is almost always present in the soil in sufficient quantities. When analysis indicates that it is needed, it may be applied in the form of magnesian lime, or refuse epsom salts, which are composed of sulphuric acid and magnesia (sulphate of magnesia).
The great care necessary concerning the use of magnesia is, not to apply too much of it, it being, when in excess, as has been previously remarked, injurious to the fertility of the soil. Some soils are hopelessly barren from the fact that they contain too much magnesia.
ACIDSSULPHURIC ACIDWhat is sulphuric acid commonly called?
How may it be used?
How does it prevent the escape of ammonia?
Sulphuric acid is a very important constituent of vegetable ashes, especially of oats and the root-crops.
It is often deficient in the soil, particularly where potatoes have been long cultivated. One of the reasons why plaster (sulphate of lime) is so beneficial to the potato crop is undoubtedly that it supplies it with sulphuric acid.
Sulphuric acid is commonly known by the name of oil vitriol, and may be purchased for agricultural purposes at a low price. It may be used in a very dilute form (weakened by mixing it with a large quantity of water) to the compost heap, where it will change the ammonia to a sulphate as soon as formed, and thus prevent its loss, as the sulphate of ammonia is not volatile; and, being soluble in water, is useful to plants. Some idea of the value of this compound may be formed from the fact that manufacturers of manures are willing to pay seven cents per lb., or even more, for sulphate of ammonia, to insure the success of their fertilizers. Notwithstanding this, many farmers persist in throwing away hundreds of pounds of ammonia every year, as a tax for their ignorance (or indolence), while a small tax in money—not more valuable, nor more necessary to their success—for the support of common schools, and the better education of the young, is too often unwillingly paid.
What is the effect of using too much sulphuric acid?
If a tumbler full of sulphuric acid (costing a few cents), be thrown into the tank of the compost heap once a month, the benefit to the manure would be very great.
Where a deficiency of sulphuric acid in the soil is indicated by analysis, it may be supplied in this way, or by the use of plaster or refuse epsom salts.
Care is necessary that too much sulphuric acid be not used, as it would prevent the proper decomposition of manures, and would induce a growth of sorrel in the soil by making it sour.
In many instances, it will be found profitable to use sulphuric acid in the manufacture of super-phosphate of lime (as directed under the head of 'phosphoric acid,') thus making it perform the double purpose of preparing an available form of phosphate, and of supplying sulphur and sulphuric acid to the plant.
PHOSPHORIC ACIDHow large a part of the ashes of grain consists of phosphoric acid?
Of what other substances does it form a leading ingredient?
How many pounds of sulphuric acid are contained in one hundred bushels of wheat?
We come now to the consideration of one of the most important of all subjects connected with agriculture, that is, phosphoric acid.
Phosphoric acid, forming about one half of the ashes of wheat, rye, corn, buckwheat, and oats; nearly the same proportion of those of barley, peas, beans and linseed; an important ingredient of the ashes of potatoes and turnips; one quarter of the ash of milk and a large proportion of the bones of animals, often exists in the soil in the proportion of only about one or two pounds in a thousand. The cultivation of our whole country has been such, as to take away the phosphoric acid from the soil without returning it, except in very minute quantities. Every hundred bushels of wheat sold contains (and removes permanently from the soil) about sixty pounds of phosphoric acid. Other grains, as well as the root crops and grasses, remove likewise a large quantity of it. It has been said by a contemporary writer, that for each cow kept on a pasture through the summer, there is carried off in veal, butter and cheese, not less than fifty lbs. of phosphate of lime (bone-earth) on an average. This would be one thousand lbs. for twenty cows; and it shows clearly why old dairy pastures become so exhausted of this substance, that they will no longer produce those nutritious grasses, which are favorable to butter and cheese-making.
How much phosphate of lime will twenty cows remove from a pasture during a summer?
What has this removal of phosphate of lime occasioned?
How have the Genesee and Mohawk valleys been affected by this removal of phosphoric acid?
That this removal of the most valuable constituent of the soil, has been the cause of more exhaustion of farms, and more emigration, in search of fertile districts, than any other single effect of injudicious farming, is a fact which multiplied instances most clearly prove.
It is stated that the Genesee and Mohawk valleys, which once produced an average of thirty-five or forty bushels of wheat, per acre, have since been reduced in their average production to twelve and a half bushels. Hundreds of similar cases might be stated; and in a large majority of these, could the cause of the impoverishment be ascertained, it would be found to be the removal of the phosphoric acid from the soil.
How may this devastation be arrested?
Is any soil inexhaustible?
What is usually the best source from which to obtain phosphoric acid?
The evident tendency of cultivation being to continue this murderous system, and to prey upon the vital strength of the country, it is necessary to take such measures as will arrest the outflow of this valuable material. This can never be fully accomplished until laws shall be made preventing the wastes of cities and towns. Such laws have existed for a long time in China, and have doubtlessly been the secret of the long subsistence and present prosperity of the millions of people inhabiting that country.
We have, nevertheless, a means of restoring to fertility many of our worn-out lands, and preserving our fertile fields from so rapid impoverishment as they are now suffering. Many suppose that soils which produce good crops, year after year, are inexhaustible, but time will prove to the contrary. They may possess a sufficiently large stock of phosphoric acid, and other constituents of plants, to last a long time, but when that stock becomes so reduced, that there is not enough left for the uses of full crops, the productive power of the soil will yearly decrease, until it becomes worthless. It may last a long time, a century, or even more, but as long as the system is—to remove every thing, and return nothing,—the fate of the most fertile soil is evident.
The source mentioned, from which to obtain phosphoric acid, is the bones of animals. These contain large quantities of phosphate of lime. They are the receptacles which collect nearly all of the phosphates in crops, which are fed to animals, and are not returned in their excrements. For the grain, etc., sent out of the country, there is no way to be repaid except by the importation of this material; but, all that is fed to animals, or to human beings, may, if a proper use be made of their excrement, and of their bones after death, be returned to the soil. With the treatment of animal excrements we are already familiar, and we will now turn our attention to the subject of
BONESOf what do dried bones consist?
What is the organic matter of bones?
The inorganic?
What can you say of the use of whole bones?
Bones consist, when dried, of about one third organic matter, and two thirds inorganic matter.
The organic matter consists chiefly of gelatine—a compound containing nitrogen.
The inorganic part is chiefly phosphate of lime.
Hence, we see that bones are excellent, as both organic and mineral manure. The organic part, containing nitrogen, forms ammonia, and the inorganic part supplies the much needed phosphoric acid to the soil.
Liebig says that, as a producer of ammonia, 100 lbs. of dry bones are equivalent to 250 lbs. of human urine.
How does the value of bone dust compare with that of broken bones?
What is the reason of the superiority of bone dust?
How is bone-black made?
Of what does it consist?
Bones are applied to the soil in almost every conceivable form. Whole bones are often used in very large quantities; their action, however, is extremely slow, and it is never advisable to use bones in this form.
Ten bushels of bones, finely ground, will produce larger results, during the current ten years after application, than would ensue from the use of one hundred bushels merely broken, not because the dust contains more fertilizing matter than the whole bones, but because that which it does contain is in a much more available condition. It ferments readily, and produces ammonia, while the ashy parts are exposed to the action of roots.
Should farmers burn bones before using them?
How would you compost bones with ashes?
In what way would you prevent the escape of ammonia?
Bone-black. If bones are burned in retorts, or otherwise protected from the atmosphere, their organic matter will all be driven off, except the carbon, which not being supplied with oxygen cannot escape. In this form bones are called ivory black, or bone-black. It consists of the inorganic matter, and the carbon of the bones. The nitrogen having been expelled it can make no ammonia, and thus far the original value of bones is reduced by burning; that is, one ton of bones contains more fertilizing matter before, than after burning; but one ton of bone black is more valuable than one ton of raw bones, as the carbon is retained in a good form to act as an absorbent in the soil, while the whole may be crushed or ground much more easily than before being burned. This means of pulverizing bones is adopted by manufacturers, who replace the ammonia in the form of guano, or otherwise; but it is not to be recommended for the use of farmers, who should not lose the ammonia, forming a part of bones, more than that of other manure.
Composting bones with ashes is a good means of securing their decomposition. They should be placed in a water-tight vessel (such as a cask); first, three or four inches of bones, then the same quantity of strong unleached wood ashes, continuing these alternate layers until the cask is full, and keeping them always wet. If they become too dry they will throw off an offensive odor, accompanied by the escape of ammonia, and consequent loss of value. In about one year, the whole mass of bones (except, perhaps, those at the top) will be softened, so that they may be easily crushed, and they are in a good condition for manuring. The ashes are, in themselves, valuable, and this compost is excellent for many crops, particularly for Indian corn. A little dilute sulphuric acid, occasionally sprinkled on the upper part of the matter in the cask, will prevent the escape of the ammonia.
What is the effect of boiling bones under pressure?
How is super-phosphate of lime made?
Describe the composition of phosphate of lime, and the chemical changes which take place in altering it to super-phosphate of lime.
Boiling bones under pressure, whereby their gelatine is dissolved away, and the inorganic matter left in an available condition, from its softness, is a very good way of rendering them useful; but, as it requires, among other things, a steam boiler, it is hardly probable that it will be largely adopted by farmers of limited means.
Any or all of these methods are good, but bones cannot be used with true economy, except by changing their inorganic matter into
SUPER-PHOSPHATE OF LIMESuper-phosphate of lime is made by treating phosphate of lime, or the ashes of bones, with sulphuric acid.
Phosphate of lime, as it exists in bones, consists of one atom of phosphoric acid and three atoms of lime. It may be represented as
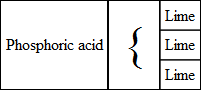
By adding a proper quantity of sulphuric acid with this, it becomes super-phosphate of lime; that is, the same amount of phosphoric acid, with a smaller proportion of lime (or a super-abundance of phosphoric acid), the sulphuric acid, taking two atoms of lime away from the compound, combined with it making sulphate of lime (plaster). The changes may be thus represented.
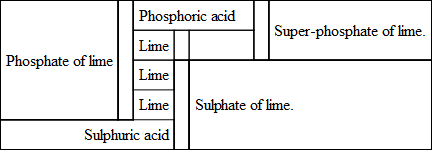
Super-phosphate of lime may be made from whole bones, bone dust, bone-black, or from the pure ashes of bones.
How should sulphuric acid be applied to whole bones?
What is the necessity for so large an amount of water?
The process of making it from whole bones is slow and troublesome, as it requires a long time for the effect to diffuse itself through the whole mass of a large bone. When it is made in this way, the bones should be dry, and the acid should be diluted in many times its bulk of water, and should be applied to the bones (which may be placed in a suitable cask, with a spiggot at the bottom), in quantities sufficient to cover them, about once in ten days; and at the end of that time, one half of the liquid should be drawn off by the spiggot. This liquid is a solution of super-phosphate of lime, containing sulphate of lime, and may be applied to the soil in a liquid form, or through the medium of a compost heap. The object of using so much water is to prevent an incrustation of sulphate of lime on the surfaces of the bones, this must be removed by stirring the mass, which allows the next application of acid to act directly on the phosphate remaining. The amount of acid required is about 50 or 60 lbs. to each 100 lbs. of bones. The gelatine will remain after the phosphate is all dissolved, and may be composted with muck, or plowed under the soil, where it will form ammonia.
May less water be employed in making super-phosphate from bone dust or crushed bones?
Bone dust, or crushed bones, may be much more easily changed to the desired condition, as the surface exposed is much greater, and the acid can act more generally throughout the whole mass. The amount of acid required is the same as in the other case, but it may be used stronger, two or three times its bulk of water being sufficient, if the bones are finely ground or crushed—more or less water should be used according to the fineness of the bones. The time occupied will also be much less, and the result of the operation will be in better condition for manure.
Bones may be made fine enough for this operation, either by grinding, etc., or by boiling under pressure, as previously described; indeed, by whatever method bones are pulverized, they should always be treated with sulphuric acid before being applied to the soil, as this will more than double their value for immediate use.
Bone-black is chiefly used by manufacturers of super-phosphate of lime, who treat it with acid the same as has been directed above, only that they grind the black very finely before applying the acid.
What other forms of bones may be used in making super-phosphate of lime?
Why is super-phosphate of lime a better fertilizer than phosphate of lime?
What can you say of the lasting manures?
Bone ashes, or bones burned to whiteness, may be similarly treated. Indeed, in all of the forms of bones here described, the phosphate of lime remains unaltered, as it is indestructible by heat; the differences of composition are only in the admixture of organic constituents.
The reason why super-phosphate of lime is so much better than phosphate, may be easily explained. The phosphate is very slowly soluble in water, and consequently furnishes food to plants slowly. A piece of bone as large as a pea may lie in the soil for years without being all consumed; consequently, it will be years before its value is returned, and it pays no interest on its cost while lying there. The super-phosphate dissolves very rapidly and furnishes food for plants with equal facility; hence its much greater value as a manure.
It is true that the phosphate is the most lasting manure; but, once for all, let us caution farmers against considering this a virtue in mineral manures, or in organic manures either, when used on soils containing the proper absorbents of ammonia. They are lasting, only in proportion as they are lazy. Manures are worthless unless they are in condition to be immediately used. The farmer who wishes his manures to last in the soil, and to lose their use, may be justly compared with the miser, who buries his gold and silver in the ground for the satisfaction of knowing that he owns it. It is an old and a true saying that "a nimble sixpence is better than a slow shilling."
IMPROVED SUPER-PHOSPHATE OF LIMEWhat are the ingredients of the improved super-phosphate of lime?
To show the manner in which super-phosphate of lime is perfected, and rendered the best manure for general uses, which has yet been made, containing large quantities of phosphoric acid and a good supply of ammonia,—hereby covering the two leading deficiencies in a majority of soils, it may be well to explain the composition of the improved super-phosphate of lime invented by Prof. Mapes.
This manure consists of the following ingredients in the proportions named:—

Explain the uses of these different constituents.
What is nitrogenized phosphate?
The sulphuric acid has the before-mentioned effect on the bone-black, and fixes the ammonia of the guano by changing it to a sulphate. The twenty pounds of sulphate of ammonia added increase the amount, so as to furnish nitrogen to plants in sufficient quantities to give them energy, and induce them to take up the super-phosphate of lime in the manure more readily than would be done, were there not a sufficient supply of ammonia in the soil.
The addition of the guano, which contains all of the elements of fertility, and many of them in considerable quantities, renders the manure of a more general character, and enables it to produce very large crops of almost any kind, while it assists in fortifying the soil in what is usually its weakest point—phosphoric acid.
Prof. Mapes has more recently invented a new fertilizer called nitrogenized super-phosphate of lime, composed of the improved super-phosphate of lime and blood, dried and ground before mixture, in equal proportions. This manure, from its highly nitrogenous character, theoretically surpasses all others, and probably will be found in practice to have great value; its cost will be rather greater than guano.


