
Полная версия
The Fontana History of Chemistry
Ironically, the growth of nineteenth-century chemistry encouraged a revival of alchemical speculation. Dalton’s reintroduction of atomism, the scepticism expressed towards the growing number of chemical elements (chapter 4), the discoveries of spectroscopists and the regularities of the periodic table (chapter 9), all suggested the possibility of transmutation. Although the possibility was given respectability by Rutherford’s and Soddy’s work on radioactivity at the beginning of the twentieth century and physically realized on an atomic scale in the 1930s, it had earlier led in the 1860s to ‘hyperchemistry’. We must not be surprised, therefore, to find gold transmutation stories occurring during even the most positivistic periods of Victorian science. During the 1860s, Chemical News (chapter 12) attributed the high price of bismuth on the metal market to a vogue for transmutation experiments. This was connected to a daring swindle perpetrated on the London stock-market by a Hungarian refugee, Nicholas Papaffy. Papaffy duped large numbers of investors into promoting a method for transforming bismuth and aluminium (then a new and expensive metal) into silver. This followed from a successful public demonstration at a bullion works in the classic tradition of Jonson’s Subtle. Needless to say, after trading offices were opened in Leadenhall Street, Papaffy decamped with an advance of £40 000 from the company. Nor was the American government less gullible. In 1897 an Irish – American metallurgist, Stephen Emmens, sold gold ingots to the US Assay Office that he claimed to have made from silver by his ‘Argentaurum Process’.
In France during the same period, hyperchemistry enjoyed the support of an Association Alchimique de France to which the Swedish playwright, August Strindberg, subscribed, and which influenced Madame Blavatsky’s ‘scientific’ writings for the theosophists and inspired the English composer, Cyril Scott (1879–1970), to compose the opera The Alchemist in 1925. The occult interest in alchemy has continued to the present day and has been given academic respectability since 1985 through the publication of the international scholarly review, Aries, a biannual devoted to the review of the history of esotericism, Hermeticism, theosophy, freemasonry, the Kabbalah and alchemy. Today, booksellers catalogue alchemy under ‘Occultism’ and not ‘History of Science’, while Ambix, the academic mouthpiece of the Society for the History of Alchemy and Chemistry (founded 1937) continues to receive occultist literature for review, as well as the occasional letter pressing its editor for ‘the secret of secrets’.
In 1980, at the phenomenal cost of $10 000, a bismuth sample was transmuted into one-billionth of a cent’s worth of gold by means of a particle accelerator at the Lawrence Laboratory of the University of California at Berkeley. The ‘value’ of the experiment is underlined in Frederick Soddy’s ironic remark some sixty years before9:
If man ever achieves this further control over Nature, it is quite certain that the last thing he would want to do would be to turn lead or mercury into gold – for the sake of gold. The energy that would be liberated, if the control of these sub-atomic processes were possible as in the control of ordinary chemical changes, such as combustion, would far exceed in importance and value the gold.
2 The Sceptical Chymist
I see not why we must needs believe that there are any primogeneal and simple bodies, of which, as of pre-existent elements, nature is obliged to compound all others. Nor do I see why we may not conceive that she may produce the bodies accounted mixt out of one another by variously altering and contriving their minute parts, without resolving the matter into any such simple and homogeneous substances as are pretended.
(ROBERT BOYLE, The Sceptical Chymist, 1661)
The phrase ‘The Scientific Revolution’ conjures up a rebellion against Greek authority in astronomy and dynamics, and physics in general. It reminds us of names like Copernicus, Kepler, Galileo, Harvey, Descartes, Bacon and Newton. Chemists’ names are missing. Indeed, a sixteenth- and seventeenth-century revolution in chemical understanding does not readily spring to mind. What was there to rebel against or to revolutionize? Was there a new chemical way of looking at substances in the seventeenth century that in any way paralleled the new physical way?
The historian’s reply has usually been a negative one, with the rider that chemistry developed much later than either astronomy or physics or anatomy and physiology; and that chemistry did not become a science until the eighteenth century. Its revolution was carried out by Lavoisier.
Whether or not this was the case, it can be agreed that chemistry presented the early natural philosopher with peculiarly difficult problems. The sheer complexity of most of the chemical materials with which chemists commonly worked can be seen, with hindsight, to have inevitably made generalizations extremely difficult. Chemists were considering with equal ardour the chemical components of the human and animal body, and of plants and minerals, the procedures of metallurgy, pottery, vinegar, acid and glass manufacture, as well as, in some quarters, abstractions like the philosopher’s stone and the elixir of life. There was no universally agreed chemical language, no convenient compartmentalization of substances into organic and inorganic, into solids, liquids and gases, or into acids, bases and salts; and no concept of purity. For example, when Wilhelm Homberg (1652–1715) ‘analysed’ ordinary sulphur in 1703, he obtained an acid salt, an earth, some fatty matter and some copper metal.
But perhaps the greatest stumbling block to the further development of chemistry was a case of insufficient analysis – there was a complete absence of a knowledge or concept of the gaseous state of matter. Chemistry remained a two-dimensional science, which studied, and only had equipment and apparatus to handle, solids and liquids.
This does not mean that chemistry lacked organization, for there were any number of grand theories that brought order and classification to this complicated subject. The problem with these organizational theories was not only their mutual inconsistency, but the fact that by the 1660s they looked old-fashioned and part of the pre-revolutionary landscape that astronomers and physicists had moved away from. To many natural philosophers, therefore, chemistry seemed tainted; it was an occult or pseudo-science that was beyond the pale of rational discourse.
This was where Boyle came in, for he devoted his life to bringing chemistry to the attention of natural philosophers as a subject worthy of their closest and honest attention. His intention was to ‘begat a good understanding betwixt the chymists and the mechanical philosophers’. In order to do this, he had to show, among other things, that the three or four traditional explanations of chemical phenomena lacked credibility and that a better explanation lay in the revived corpuscular philosophy.
PARACELSIANISM
Philippus Aureolus Theophrastus Bombast von Hohenheim (1493–1541), who rechristened himself Paracelsus in order to indicate his superiority to the second-century Roman medical writer, Celsus, was born near Zurich, then still nominally part of the Holy Roman empire and under Austrian domination. At the age of twenty-one, on the advice of his physician father, he visited the mines and metallurgical workshops in the Tyrol where he studied metallurgy and alchemy. After claiming a medical degree from Ferrara in Italy, Paracelsus became Medical Officer of Health at Basel, a position he was forced to leave in an undignified manner two years later after his abusive and bombastic manner had offended public opinion. Thereafter, he became a rolling stone, restlessly traversing the roads and countries of war-torn Europe, associating with physicians, alchemists, astrologers, apothecaries, miners, gypsies and the adepts of the occult.
It is easy to see why he offended. Not only did he lecture in German instead of Latin, an unorthodox behaviour for a physician, but he publicly burned the works of Galen and Avicenna to show his contempt for orthodox medical opinion – a ceremony that was to be repeated by Lavoisier and his wife 250 years later.
If your physicians only knew that their prince Galen … was sticking in Hell, from whence he has sent letters to me, they would make the sign of the cross upon themselves with a fox’s tail. In the same way your Avicenna sits in the vestible of the infernal portal.
Come then and listen, impostors who prevail only by the authority of your high positions! After my death, my disciples will burst forth and drag you to the light, and shall expose your dirty drugs, wherewith up to this time you have compassed the death of princes .… Woe for your necks on the day of judgement! I know that the monarchy will be mine. Mine too will be the honour and the glory. Not that I praise myself: Nature praises me.
Is this rhetoric or the ravings of a lunatic?
Not surprisingly, contemporary estimates of Paracelsus varied tremendously. An opinion that ‘he lived like a pig, looked like a drover, found his greatest enjoyment in the company of the most dissolute and lowest rabble, and throughout his glorious life he was generally drunk’, may be contrasted with his pupils’ expressions, ‘the noble and beloved monarch’, ‘the German Hermes’ and ‘our dear Preceptor and King of Arts’. What did this contradictory, bewildering figure do for chemistry? What did he teach?
Most of his writings were only published posthumously and there has always been controversy between historians who accept only the ‘rational’ writings as genuine and those who view his eclectic mixture of rationalism, empiricism, Neoplatonic occultism and mysticism as the genuine Paracelsus. Although he definitely subscribed to alchemy, i.e. to the doctrine of transmutation, ‘alchemy’ had a wider meaning for Paracelsus. It entailed carrying ‘to its end something that [had] not yet been completed’. It was any process in Nature in which substances worked or metamorphosed to a new end, and thus included cookery and the chemical arts as well as physiological processes such as digestion.
This widened sense of the word was to be reflected explicitly in what has been described as the first chemistry textbook, the Alchemia published by the Lutheran humanist, Andreas Libavius (1540–1616), in 1597, though, as we shall see, Libavius was contemptuous of Paracelsus. For Paracelsus, chemistry was the key subject for unveiling the secrets of a universe that had been created by a chemist and operated by chemical laws. The views of Aristotle and Galen were those of heathens and heretics and had to be replaced by an empiricism that was controlled by Christian and Neoplatonic insights. Paracelsus and his followers, such as Ostwald Croll in his ‘royal chemistry’, the Basilica Chymica (1609), often made much of the story of creation in Genesis, which they interpreted as a chemical allegory. Paracelsianism thereby came to share many of the attributes of esoteric alchemy in which ‘the art’ was essentially a personal religious avocation.
On the other hand, Paracelsus saw himself essentially as a medical reformer, as someone destined to refute age-old teachings and to base medical practice on what he claimed were more effective mineral medicines. He taught that the principal aim of medicine should be the preparation of arcana, most of which turn out to be chemical, inorganic remedies as opposed to the herbal, organic medicines derived from Greco-Roman medicine. The arcana would destroy and eliminate poisons produced by disease, which itself was due to the putrefaction of the ‘excrements’ produced in any ‘chemical’ process. Diseases were therefore specific, as the new pandemic of syphilis then sweeping Europe suggested, and were to be cured by specific arcana.
Paracelsus taught that macrocosm (the heavens) and microcosm (the earth and all its creatures) were linked together. The heavens contained both visible and invisible stars (astra) that descended to impregnate the matter of the microcosm, conferring on each body the specific form and properties that directed its growth and development. Like acted upon like. The task of the chemist was, by experiment and knowledge of macrocosmic – microcosmic correspondences (the doctrine of signatures), to determine an astral essence or specific virtue capable of treating a disease. To isolate the remedy, the alchemist-physician had to separate the pure essence from the impure, by fire and distillation. Here, Paracelsus owed much to the medieval technology of distillers and to the writings of John of Rupescissa in the fourteenth century. The latter had identified Aristotle’s fifth, heavenly element, the ether, as a quintessence that could be distilled from plants. Paracelsus and his followers were, however, rather more interested in the inorganic salts remaining after distillation than in the distillates themselves.
In this way Paracelsus initiated a new study he called ‘iatrochemistry’, which invoked chemistry to the aid of medicine. Whereas the Paracelsians were individualistic in their pantheistic interpretation of Nature, regarding chemical knowledge as incommunicable except between and through the inspiration proper to a magus, Libavius and the textbook writers who followed him argued that chemistry could be learned by all in the classroom, provided it was put into a methodical form. This construction of a pedagogical discipline involved the classification of laboratory techniques and operations and the establishment of a standardized language of chemical substances. Progress in chemistry, or in any science, would come only from a collective endeavour to combine the subjective, and possibly unreliable, contributions of individuals after subjecting them to peer review and measuring each one critically against past wisdom and experience.
Iatrochemical doctrines became extremely popular during the seventeenth century, and not unlinked with this was a rise in the social status of the apothecary. Both in Britain and on the Continent there was a compromise in which chemical remedies were adopted without commitment to the Paracelsian cosmology. Didactically acquired knowledge of iatrochemistry gave these medical practitioners (who in Britain were to become the general practitioners of the nineteenth and twentieth century) a base upon which they could branch out into their own medical practice and away from the control of university-educated physicians. The need for self-advertisement encouraged them to teach iatrochemical practice and to introduce inorganic remedies into the pharmacopoeia. They were therefore less secretive than the alchemists. Because they wanted to find and prepare useful medical remedies, they were keen to know how to recognize and prepare definite chemical substances with repeatable properties. In teaching their subject, what was wanted was a good textbook, which would provide clear and simple recipes for the preparation of their drugs, with clear unambiguous names for their substances and adequate instructions on the making and use of apparatus for the preparations. Theory could play second fiddle to practice.
Iatrochemistry became very much a French art and here the subject was helped in Paris by the existence of chemical instruction at the Jardin du Roi. Beginning with Jean Beguin’s Tyrocinium Chymicum in 1610, which plagiarized a good deal from Libavius’ Alchemia, each successive professor, Étienne de Clave, Christopher Glaser and Nicholas Lemery, composed a textbook for the instruction of the apothecary’s apprentices who flocked to their annual lectures. Many of these texts went into other languages, including Latin and English. By 1675, when Lemery published his Cours de Chimie, a textbook tradition had been firmly established as part of didactic chemistry and which considerably aided the establishment of chemistry as a discipline. Some historians of chemistry believe that this formulation of chemistry as a scholarly, didactic discipline, which began with Libavius well before the establishment of the mechanical philosophy, was far more significant than the latter for the creation of modern chemistry.
In chemical theory, Paracelsus introduced the doctrine of the tria prima, or the three principles. Medical substances, he said, were ultimately composed from the four Aristotelian elements, which formed the receptacles or matrices for the universal qualities of a trinity of primary bodies he called salt (body), sulphur (soul) and mercury (spirit).
The world is as God created it. He founded this primordial body on the trinity of mercury, sulphur and salt and these are the three substances of which the complete body consists. For they form everything that lies in the four elements, they bear them all the forces and faculties of perishable things.
The doctrine of the tria prima was clearly an extension of the Arabic sulphur – mercury theory of metals applied to all materials whether metallic, non-metallic, animal or vegetable, and given body by the addition of a third principle, salt.
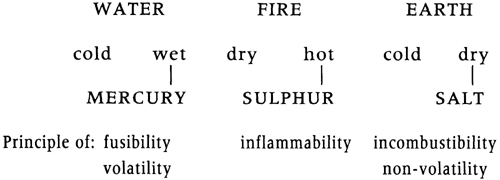
This theory of composition, which essentially explained gross properties by hypothetical property-bearing constituents, rapidly replaced the old sulphur – mercury theory, though not the Aristotelian four elements. Paracelsus was happy to use Aristotle’s example of the analysis of wood by destructive distillation to justify the tria prima theory. Smoke was the volatile portion, mercury; the light and glow of the fire demonstrated the presence of sulphur; and the incombustible, non-volatile ash remaining was the salt. Water was included within the mercury principle, which explained the cohesion of bodies.
Van Helmont begged to differ and provided a simpler, and supposedly more empirical, alternative theory of composition.
HELMONTIANISM
Iatrochemistry came to fruition in the work of a Flemish nobleman, Joan-Baptista van Helmont (1577–1644). Present-day Belgium was then under Spanish control. In 1625, as a consequence of Helmont’s controversial advocation of ‘weapon salve’ treatment in which a weapon, and not a wound, was treated, he was denounced as a heretic by the Spanish Inquisition and spent the remainder of his life, like Galileo, under house arrest. As with Paracelsus, it was van Helmont’s posthumous writings that brought his name to fame and exerted a considerable influence upon seventeenth-century natural philosophers like Boyle and Newton. This influence was firmly established after 1648 with the posthumous publication of his Ortus Medicinae, which was issued in English in 1662 as Oriatricke or Physick Refined. Helmont, who claimed to have witnessed a successful transmutation of a base metal into gold, was a disciple of Paracelsus and an iatrochemist. However, like any good disciple, he modified, interpreted and disagreed with his master’s doctrines considerably.
After studying several areas of natural philosophy, he chose medicine and chemistry for his career, calling himself a ‘philosopher by fire’. He was strongly anti-Aristotelian, one facet of which was that he refused to accept the four-element theory. But neither was he able to accept Paracelsus’ tria prima. To simplify a rather complex philosophy, we can say that according to van Helmont there were two first beginnings of bodies: water and an active, organizing principle, or ‘ferment’, which moulded the various forms and properties of substances. This return to a unitary theory of matter was influenced by his interpretation of Genesis, for water, together with the heavens and the earth, had been formed on the first day.
In more detail, he imagined that there were two ultimate elements, air and water. Air was, however, purely a physical medium, which did not participate in transmutations, whereas water could be moulded into the rich variety of substances found on the earth. Van Helmont did not consider fire to be a material element, but a transforming agent. As for earth, from his experimental observations, he believed that this was created by the action of ferments upon water.
The first beginnings of bodies, and of corporeal causes, are two, and no more. They are surely the element water, from which bodies are fashioned, and the ferment.
As we have already seen in the introduction, the justification for this belief was an interesting, quantitative growth experiment with a young willow tree. Additional supporting evidence came from the fact that fish were nourished ‘solely’ by water, that seashells were found on dry land, and that solid bodies could be transformed into ‘savoury waters’, that is, into solution. In the latter case, Helmont took a weighed amount of sand, and fused it with excess alkali to form water-glass, which liquefied on standing in air. Here was a palpable demonstration of the reconversion of earth back into water. More remarkably, this ‘water’ could be reconverted back to ‘earth’ by treatment with acid, when the silica sand recovered was found to have the same weight as the starting material.
There are a number of interesting features about these experiments and Helmont’s reasoning. Their most important feature is not that Helmont misinterpreted his observations because he ignored the role of air, but that they were quantitative. The experiments were also controlled. In the willow tree experiment, Helmont covered the vessel so as to prevent dust contamination, which might have affected the result. Similarly, he dried the earth beforehand and used only distilled water. He clearly had thought about the experiment and possible objections that might be raised against his conclusions because of the way the experiment had been designed. All this was the hallmark of the experimental method that was to lead to the transformation of chemistry. In addition, it is noticeable that he implicitly assumed that matter was conserved in any changes it underwent. When metals were dissolved in acids they were not destroyed, but were recoverable weight for weight. Helmont also postulated the existence of an alcahest, or universal solvent, which had the property of turning things back into water. Much time and effort was spent by contemporary chemists, including Robert Boyle, in trying to identify this mysterious solvent.
There is a further item of interest to be found in Helmont’s writings. Since air could not be turned into water, he accepted it as a separate element. However, his keen interest was awakened by the ‘air-like’ substances that were frequently evolved during chemical reactions. Helmont identified these fuliginous effluvia as ‘gases’, from a Greek word for ‘chaos’ that Paracelsus had ascribed to air in another connection. Where did these uncontrollable, dangerous materials fit in Helmont’s ontology?
Gases were simply water, not air, for any matter carried into the atmosphere was turned into gas by the cold and ‘death’ of its ferments. A gas was chaos because it bore no form. A gas might also condense to a vapour and fall as rain under the influence of blas, a term that did not stay in chemical language, and which Helmont coined to refer to a kind of ‘gravitational’, astral influence or power that caused motion and change throughout the universe.
In a typical gas experiment, Helmont heated 62 lb (28 kg) of charcoal in air and was left with 1 lb (2.2 kg) of ash, the rest having disappeared as ‘spiritus sylvester’ or wild spirit. When charcoal was heated in a sealed vessel, combustion would either not occur, or would occur with violence as the spirit escaped from the exploding vessel. This disruptive experience led to Helmont’s definition of gas:
This spirit, hitherto unknown, which can neither be retained in vessels nor reduced to a visible body … I call by the new name gas.




