
Полная версия
The Tangled Tree: A Radical New History of Life
Methanogens: their name derives from an odd aspect of their biochemistry, producing methane as a byproduct while metabolizing hydrogen and carbon dioxide in environments lacking oxygen. To say it more plainly, these bugs generate swamp gas in muddy wetlands, from which it bubbles up, and similar gas in the bellies of cows, whence it emerges by belch and fart. Certain methanogens also thrive beneath the Greenland ice cap, deep in the oceans, and in other extreme environments, such as hot desert soils. Despite these shared metabolic traits, Ralph Wolfe advised Woese, there was an odd discontinuity among the assemblage of methanogens—discontinuity in terms of their shapes. Some were cocci (spherical), some were bacilli (rod shaped). Since the cocci and the bacilli were considered two distinct kinds of bacteria, microbiologists had been puzzled about how to classify the methanogens—together by metabolism or separately by shape. That conundrum captured Woese’s interest.
Having told me this much, and more, Ken Luehrsen finished our conversation and sent me away with some gifts. One was a black-and-white print of a photo he took in the mid-1970s, a snapshot, showing Woese at his light board, engrossed before a pattern of dark spots, with a handful of felt-tip pens for color coding what he saw, a pencil for data registry behind his right ear. Luehrsen’s other gift was a single yellowing sheet—not a copy, the original—from his own notebook of the time. It was a catalog of fragments from an organism, more of those telling blurts of the four coding letters, neatly recorded in two columns. UCUCG. CAAG. GGGAAU, and dozens more. At the top, also hand lettered, an abbreviation indicated the name of the organism as it was known at the time: Methanobacterium ruminantium. Later, I realized that, notwithstanding the name, this was no bacterium. Luehrsen had given me the genetic rap sheet on a separate form of life.
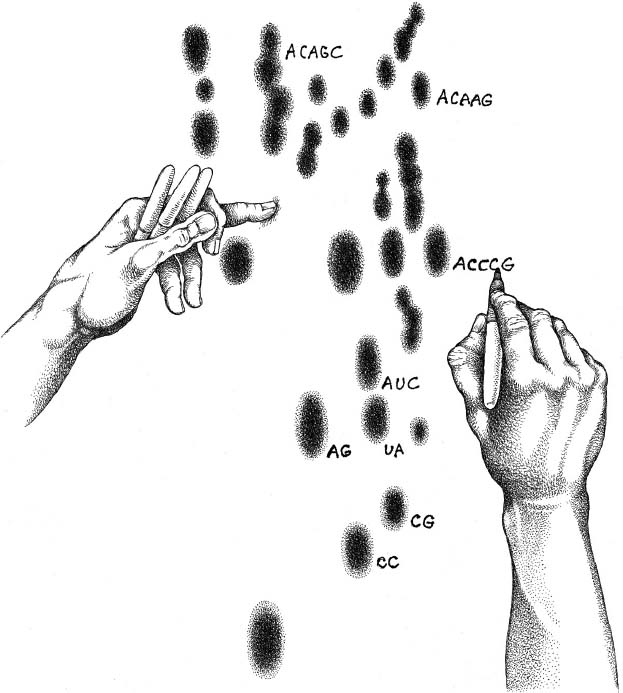
Annotating RNA fragments on a “fingerprint” film.
16
How do you classify the methanogens? Where do they fit on the tree of life? To what other little bugs are they most closely related? Those questions, which Woese and his colleagues were asking themselves in the mid-1970s, fell within the scope of an important discipline with a dry name: bacterial taxonomy. That’s the enterprise of sorting bacteria into nested groups: species, genera, families, etcetera. You name something Methanobacterium ruminantium, and then where do you put it?
This may sound like an exercise in arcana, a marginal activity of risible triviality beside which stamp collecting looks like an adventure sport. Bacteria are tiny, relatively simple, invisible. But if being invisible made things unimportant, gravity and microwaves would be unimportant too. It’s useful to recall that most life-forms on Earth are microbial, that they determine the conditions of existence for the rest of us, and that even the human body contains at least as many microbial cells (those tiny passengers that live in your gut, on your skin, in the follicles of your eyelashes, and elsewhere) as human cells. Your environment is highly microbial too. Your food. The air you breathe. Microbes run the world, and a very large portion of those microbes are bacteria. Some of them serve as helpful partners of humanity. Some are benign. Some are rapacious, ready to poison your blood, fill your lungs, kill you. So it’s no small matter, telling one bacterium from another.
Scientists once believed it might be possible to do this from visual evidence obtained through a microscope. They even presumed that the concept of species, as understood for animals and plants and fungi, could be applied to bacteria. These were useful simplifications in their era—like the simplifications of Newtonian physics, before correction by Einstein—but that era was a long time ago.
The early hero in the field was a man named Ferdinand Julius Cohn, a botanist and microbiologist at the University of Breslau (now Wrocław, Poland) during the late nineteenth century. Cohn is an appealing figure, and only partly because his important contributions have been overshadowed by those of better-remembered contemporaries whose accomplishments were more practical and dramatic: Louis Pasteur, Robert Koch, Joseph Lister. They worked on disease, agriculture, and wine. Cohn worked mainly on describing and classifying microscopic organisms. No one makes Hollywood movies about bacterial taxonomists.
Cohn wasn’t the first researcher to classify bacteria, making distinctions between kinds, trying to place the whole group in its proper position on the tree of life. But his effort was more hardheaded and percipient than the others, and he did much to bring bacteriology out of a fog of confusions that had lingered for more than a century, ever since startled observers such as Leeuwenhoek had noticed these little creatures through simple microscopes. Several insights and adjustments of method helped him make progress. Microscopy improved, with better lenses and precision instruments in which they were mounted. Cohn’s lab started culturing bacteria on solid media such as slices of cooked potato, not in liquid nutrient, the old way. That allowed Cohn to choose, cultivate, and consider different strains separately. Also, he recognized that physiological and behavioral characteristics as well as structural ones could be useful for distinguishing bacterial species: How do they grow in different media? How do they move? By this time, too, Cohn had embraced Darwin’s theory of evolution, and so it made sense to him that bacterial strains might change and adapt over time. This was incremental change, very different from the sort of utter transformation—one bacterial form suddenly morphing into another—that some scientists imagined to occur. Cohn didn’t buy transformation. He saw bacteria as fundamentally stable in their identities. Finally, he published his system, dividing them into four tribes: spherical, rod shaped, filamentous, and spiral, each of which got an imposing Latinate name. Within the tribes, he drew finer distinctions, separating them into genera and species.
Not everyone in the field accepted Cohn’s classification of bacterial species or his conviction about their stable identities, and the idea of shape-shifting bacteria lingered for more than a decade. The longer judgment of science historians was good to him, as a man and a scientist, noting his “reserve” against self-promotion, his modesty, his eloquent lecturing, and his success in “disentangling almost everything that was correct and important out of a mass of confused statements on what at that time was a most difficult subject to study.” Besides arguing for the reality of bacterial species and sketching a way to classify them, Cohn did much, along with Pasteur, to kill the resilient delusion that new life-forms arise by spontaneous generation. They don’t, he showed. When bacteria seem to appear out of nowhere, it’s because they have arrived from somewhere: contamination, floating through the air, reawakening spores. Cohn’s work was “entirely modern in its character and expression,” according to an authoritative chronicler of the field, writing in 1938, “and its perusal makes one feel like passing from ancient history to modern times.” But what looked modern in 1938, of course, doesn’t look modern now.
Even the devoutly empirical Ferdinand Cohn made mistakes. For one: after all his research, he still believed, as many of his colleagues did, that bacteria belong to the kingdom of plants. So his tree of life, by later standards, was badly wrong. For another: the premise of radical transformation, one bacterial form to another, turns out to be vastly more complicated than he could imagine.
17
Chaos” was the name of the group into which Linnaeus, the great systematizer, in the 1774 edition of his Systema Naturae, had lumped Leeuwenhoek’s bacteria and other little creatures. That was a durable judgment. Even well into the twentieth century, decades after Ferdinand Cohn, experts were still arguing about whether bacterial taxonomy was a meaningful enterprise or hopelessly chaotic.
Beginning in 1923, the standard source for identifying bacteria was a thick compendium, Bergey’s Manual of Determinative Bacteriology, edited by the bacteriologist David Hendricks Bergey. But as microbiology progressed, it became clear that the Bergey’s system was vague, inconsistent, and, on some fundamentals, inaccurate. It didn’t offer a tree of bacterial life. It was only a glorified field guide. Still, other researchers who critiqued Bergey’s Manual, and then tried to improve on it, found the critiquing much easier than the improving. The task of bacterial classification was just so difficult. There was almost no fossil record of bacterial ancestors. There weren’t enough differences of external shape and internal anatomy, even as seen through powerful microscopes, to support fine distinctions. Physiological characters could also be misleading, if they reflected parallel adaptations rather than shared ancestry. What did that leave for a classifier to use? (Hint: Carl Woese would offer an answer, but not until 1977.) This conundrum came to a head in 1962, when two of the world’s leading microbiologists, C. B. van Niel and Roger Stanier, essentially threw up their hands in despair.
Van Niel was a Dutchman, educated in Delft, who in 1928 decamped to California, where he taught at a marine biological station that was part of Stanford University. His particular interests were bacterial physiology and taxonomy. Roger Stanier was a younger Canadian who became van Niel’s student, then his special protégé, then his collaborator. In 1941, when Stanier was still just twenty-five years old, he and van Niel coauthored an influential paper on bacterial classification.
That paper stood as definitive for a generation—until both authors renounced it. Stanier himself later admitted some embarrassment about it, all the more so because he had arm-twisted van Niel to sign on as coauthor—student and teacher together, although the work was mainly Stanier’s. What the paper contained, besides a pointed critique of Bergey’s Manual, was a shiny new proposal for classifying bacteria—not just a checklist or a field guide but a “natural” system reflecting their evolutionary relationships. That system divided the familiar bacteria into four major groups (as Ferdinand Cohn had done) and placed them in a kingdom of simple creatures along with just one other group: the blue-green algae.
Algae? Yes, the blue-green algae, as they were then called, had long been an ambiguous group, because they seemed to straddle the line between bacteria and plants. (This was partly what allowed Cohn to believe that all bacteria were plants—the blurry lines around blue-green algae.) Algae was a catchall term for a loose assemblage of creatures that photosynthesize, including these tiny blue-green creatures, but that didn’t mean all algae shared a single common ancestor. Did they? Stanier and van Niel said no. By their new definition of things, blue-green algae were more similar to bacteria than to other algae, and these two groups should be lumped together in a kingdom of their own, apart from everything else. Eventually they labeled such cells procaryotic—meaning “before kernel,” as I’ve mentioned—and set them in contrast to eucaryotic cells, comprising all else. (Their spellings were later corrected, from more accurate transliteration of the Greek roots, to prokaryotic and eukaryotic.) The kernel in question was a cell nucleus. Just as a bacterium doesn’t have one, neither do the creatures that were then known as blue-green algae (and are now classified as cyanobacteria). Advances in microscopy since the end of World War II, including electron microscopy, had given microbiologists a better view of those distinctions and others, making possible a fresh analysis of what a bacterium is—and what it isn’t. Stanier and van Niel offered that fresh analysis along with the prokaryote category in a new paper, published in 1962, titled “The Concept of a Bacterium.” By their lights, the “abiding intellectual scandal of bacteriology” was that no such concept had ever been clearly delineated. What was a bacterium? Um, hard to say.
They tried to correct that by placing bacteria and blue-green algae together as prokaryotes, and setting them in contrast to the alternative category, eukaryote, which encompasses all other forms of cellular life. The chief distinguishing features of a prokaryote, according to Stanier and van Niel, were: (1) no cell nucleus, (2) cell division by simple fission, rather than the elaborate process of chromosome pairing known as mitosis, and (3) a cell wall strengthened by a certain sort of latticework molecule with a fancy name, peptidoglycan. I know, it looks like the moniker of a flying reptile from the Jurassic. Forget about it for now, and when peptidoglycan comes back as an important clue toward understanding the deepest structure of the tree of life, and the twig on the branch on the limb from which we humans have sprouted, I’ll remind you.
The dichotomy between prokaryotes and eukaryotes, creatures without cell nuclei and those with, relatively simple beings and relatively complex, became a fundamental organizing principle of biology. Stanier and his two coauthors of a textbook would later say that it “probably represents the greatest single evolutionary discontinuity to be found in the present-day living world.” It was also a salubrious reminder to humans of our inescapable linkage to other creatures, including some very humble ones. We are, at the most basic level of classification, eukaryotes. So are amoebae. So are yeasts. So are jellyfish, sea cucumbers, the little parasites that cause malaria, and rhododendrons. To an average person, the gap between an amoeba and a bacterium may seem narrow (partly because most of us have never, or at least not since high school biology, looked through a microscope at either), but the prokaryote-eukaryote distinction reveals it as oceanic. You could think of the living world—and, beginning from Stanier and van Niel’s 1962 paper, biologists did think of the living world—as divided into proks and euks.
Besides putting that idea into play, “The Concept of a Bacterium” is notable for having signaled surrender, by Stanier and van Niel, in the battle of bacterial taxonomy. About this they were candid, confessional, and brusque. Ever since Leeuwenhoek, microbiologists had been seeking the best way to classify bacteria. Ever since Darwin, they had been arguing about how one bacterium was related to another. Enough was enough. “Any good biologist finds it intellectually distressing to devote his life to the study of a group that cannot be readily and satisfactorily defined.” C. B. van Niel himself had devoted forty years. He and Stanier now alluded to the “elaborate taxonomic proposal” they had published back in 1941, “which neither of us cares any longer to defend.” Never mind that. They admitted having “become sceptical about the value” of any such formal systems, or the effort spent to develop them, although they still affirmed the importance of figuring out just what the devil bacteria are.
This skepticism, this taxonomist’s despair, had been wiggling up inside van Niel for a long time. Two decades earlier, even as he was signing onto that first elaborate proposal, he had confessed his gloom to Stanier in a letter: “Many, many years ago I often went around with a sense of futility of all our (my) efforts. It made me sick to go around in the laboratory (this was in Delft) and talk and think about names and relations of microorganisms.” Was any of it real? Was there any value to putting bacteria into labeled boxes? “During those periods I would go home after a day at the lab, and wish that I might be employed somewhere as a high-school teacher.” Not that he would enjoy such teaching, he realized, but at least “it would give me some assurances that what I was doing was considered worth-while.” Nowadays we might see that as a signal of bipolar disorder, but it’s just as likely that van Niel simply viewed bacterial taxonomy with great clarity.
Under their revised spellings, prokaryote and eukaryote, those two became enshrined for a generation as the most fundamental categories of life. Eukaryotes had cell nuclei. Prokaryotes did not. That dichotomy seemed to represent, as Stanier and his coauthors had written, the greatest single evolutionary divide in the living world. There were two basic kinds of creature, the proks and the euks, and there was nothing between.
What makes this worth knowing is that Carl Woese proved it wrong.
18
As of early 1976, with Ken Luehrsen and others still helping, Woese had done his unique form of catalog analysis on samples from roughly thirty species, using differences in ribosomal RNA molecules to measure their relatedness. Most were prokaryotes, but he also looked at a few eukaryotes (which carried that slightly different molecule in their ribosomes, 18S rRNA instead of 16S), including yeast, for purposes of gross comparison. He could tell a prok from a euk just by inspecting the spots on a sheet of film. And he was eager to see those “unusual bacteria,” the methanogens, about which Ralph Wolfe had alerted him.
The tricky thing about methanogens was that, since oxygen poisoned them, they were hard to grow in a laboratory. But Wolfe’s lab team included an ingenious doctoral student, Bill Balch, who had solved that problem by devising a way to culture methanogens in pressurized aluminum tubes with black rubber stoppers, and using syringes to move things in and out. Balch gave the methanogens an atmosphere of hydrogen and carbon dioxide instead of oxygen, plus a liquid growth medium, and they thrived. Woese sent his own postdoc, a rangy young man named George Fox, trained in chemical engineering, to work with Balch on growing some of these methanogens and tagging them with radioactive phosphorus. Fox, Ken Luehrsen, and other members of the Woese lab then combined their efforts on the rest of the process: extracting the radioactive RNA, purifying it to get concentrations of 16S and 5S molecules, chopping those molecules into pieces, running the electrophoresis to separate the fragments, and printing the spots onto films. Their first methanogen carried a formal name so long (Methanobacterium thermoautotrophicum) that even Woese himself dismissed that as “a fourteen-syllable monstrosity” and preferred using a shorter label, denoting the particular laboratory strain: delta H. Examining its primary fingerprint on his light board, Woese noticed something odd.
He was practiced enough by now at reading such fingerprints that he could immediately recognize a certain pair of small fragments, common to all bacteria, that “screamed out” their membership in the prokaryotes. He looked for them on the primary film from delta H. They were missing. Intrigued but patient, he waited for the secondary fingerprint, with the fragments pulled sideways to reveal more detail. He got that from his technician several days later. On June 11, 1976, he taped the primary film up on his light board again, with the secondary now in front of him on the light table, and began trying to interpret what he saw. He intended, as usual in this stage of the process, to use the secondary film as a guide for inferring the base sequences of the fragments in the primary pattern. Apart from his board and his table, the room was dark. His face, we can imagine, reflected an eerie glow. Quickly he noticed more oddities.
The two missing fragments were still missing, but it wasn’t just that. Woese turned to a different part of the pattern, expecting to see another familiar fragment—a “signature” sequence in all prokaryotes. Not there. Instead, he found a strange fragment, a longish sequence that shouldn’t have been present at all. “What was going on?” he later recalled wondering. This methanogen rRNA just “was not feeling” prokaryotic. And the more fragments he sequenced, the less prokaryotic it felt. By this time, he knew the sequences of ribosomal RNA in bacteria so intimately that his “feel” for the molecule was a persuasive standard of normality. And something in this particular creature, delta H, was abnormal. Some bacterial fragments were appearing where expected, as expected, yes. But some others looked eukaryotic, suggesting a completely distinct form of life: a yeast, a protozoan, what? And still others were just weird. What was this RNA? he wondered, and what manner of organism did it represent? It couldn’t be from a prokaryote. It wasn’t eukaryotic. It wasn’t from Mars, because it contained too many familiar stretches of RNA code. “Then it dawned on me,” he wrote. There was “something out there”—out there in the teeming ecosystems of planet Earth, he meant—other than prokaryotes and eukaryotes. A third form of life, separate.
Woese called this, whimsically, his “out-of-biology experience.” It would be the watershed moment of his scientific life.
19
After his death in December 2012, Woese’s files of scientific correspondence, manuscripts, journal articles, and other materials went to the University of Illinois Archives to be indexed, curated, and preserved. The archives are held in several different locations, one of which is the Archives Research Center, a sort of annex, housed in an old, barnlike building of red brick on Orchard Street near the south edge of the campus. A sign in front identifies this, confusingly but historically, as the Horticulture Field Laboratory; a bank of yew bushes and a riot of hostas guard the entrance. Inside, filed neatly in thirty-four boxes that can be accessed by request, are the Carl Woese Papers. I was working there at a table one hot July afternoon, reading through letters, looking for clues about the human side of this peculiar man, when John Franch arrived, wearing a dark T-shirt and a ball cap. Franch is the assistant archivist who was sent to clean out Woese’s lab after the funeral, and who knows the material found there better than anyone else. He had heard about my interest and wanted to show me something.
He led me toward the back of the building, where the roof arches high, and unlocked a door. This was one of the “vaults,” he told me, that formerly served for storing fruit—apples in particular—from the horticultural research orchards from which Orchard Street got its name. At one point, there were 125 varieties of apple grown just behind the building, and they came in by the basket and the crate to be stored here or pressed for cider and vinegar. Beyond the door, we entered an air-conditioned room, empty of apples now but lined along its left side with tall metal shelves, along its right side with tables. The shelves held hundreds of large, flat yellow boxes—the original packaging of Kodak medical X-ray films—representing the library of Woese’s RNA sequencing fingerprints. Each box was labeled along its edge with a date and the organism whose fragments were depicted.
Across the room, some films lay on the tables, where Franch had been working over them. He showed me three large sheets, carefully taped together, forming a triptych of images. I stared at the patterns of dark spots: amoebae galloping on a plain. To me, they made no particular sense. But to Woese, they had spoken eloquently of identity, relationship, evolution. If something was odd, he would have seen it.


